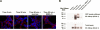Role of ubiquitylation and USP8-dependent deubiquitylation in the endocytosis and lysosomal targeting of plasma membrane KCa3.1 - PubMed (original) (raw)
Role of ubiquitylation and USP8-dependent deubiquitylation in the endocytosis and lysosomal targeting of plasma membrane KCa3.1
Corina M Balut et al. FASEB J. 2011 Nov.
Abstract
We recently demonstrated that plasma membrane KCa3.1 is rapidly endocytosed and targeted for lysosomal degradation via a Rab7- and ESCRT-dependent pathway. Herein, we assess the role of ubiquitylation in this process. Using a biotin ligase acceptor peptide (BLAP)-tagged KCa3.1, in combination with tandem ubiquitin binding entities (TUBEs), we demonstrate that KCa3.1 is polyubiquitylated following endocytosis. Hypertonic sucrose inhibited KCa3.1 endocytosis and resulted in a significant decrease in channel ubiquitylation. Inhibition of the ubiquitin-activating enzyme (E1) with UBEI-41 resulted in reduced KCa3.1 ubiquitylation and internalization. The general deubiquitylase (DUB) inhibitor, PR-619 attenuated KCa3.1 degradation, indicative of deubiquitylation being required for lysosomal delivery. Using the DUB Chip, a protein microarray containing 35 DUBs, we demonstrate a time-dependent association between KCa3.1 and USP8 following endocytosis, which was confirmed by coimmunoprecipitation. Further, overexpression of wild-type USP8 accelerates channel deubiquitylation, while either a catalytically inactive mutant USP8 or siRNA-mediated knockdown of USP8 enhanced accumulation of ubiquitylated KCa3.1, thereby inhibiting channel degradation. In summary, by combining BLAP-tagged KCa3.1 with TUBEs and DUB Chip methodologies, we demonstrate that polyubiquitylation mediates the targeting of membrane KCa3.1 to the lysosomes and also that USP8 regulates the rate of KCa3.1 degradation by deubiquitylating KCa3.1 prior to lysosomal delivery.
Figures
Figure 1.
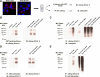
Membrane KCa3.1 is ubiquitylated during endocytic trafficking. A) At time 0 min, KCa3.1 is localized at the plasma membrane (red) and is completely endocytosed after 90 min incubation at 37°C. B) To detect ubiquitylated KCa3.1, plasma membrane channel was enzymatically biotinylated and labeled with streptavidin; cells at time 0 and 90 min were lysed in the presence of GST-TUBE2. Following pulldown, the immunoblot (IB) was probed using α-streptavidin Ab to identify BLAP-tagged KCa3.1/streptavidin. To control for nonspecific labeling, KCa3.1- expressing cells were labeled with streptavidin in the absence of enzymatic biotinylation (control lanes in all IB). No channel is detected in these controls, confirming the specificity of our labeling protocol. As shown in lane 3, endocytosed KCa3.1 is heavily ubiquitylated (time 90 min) compared with channel present at the plasma membrane (time 0 min; lane 2). Importantly, similar levels of KCa3.1 were detected for the two time points, as assessed by IB of total cell lysate (lanes 5 and 6). C) Samples were prepared and lysed as in B, then KCa3.1 was immunoprecipitated using streptavidin Ab, and the subsequent IB was probed using α-ubiquitin Ab. KCa3.1 is strongly ubiquitylated following endocytosis. D) HEK cells were doubly transfected with BLAP-KCa3.1 and HA-ubiquitin. Ubiquitin was immunoprecipitated using an anti-HA Ab and subsequently IB for streptavidin-tagged KCa3.1. E) KCa3.1 was immunoprecipitated using an anti-streptavidin antibody and the subsequent IB probed with anti-HA Ab.
Figure 2.
Ubiquitylation is required for KCa3.1 endocytosis. A) BLAP-KCa3.1 was enzymatically biotinylated at the cell surface and labeled with streptavidin-Alexa555 or streptavidin and incubated at 37°C for 90 min in the absence or presence of hypertonic sucrose, an inhibitor of endocytosis, or the ubiquitin-activating enzyme (E1) inhibitor UBEI-41. Both hypertonic sucrose and UBEI-41 dramatically reduced the internalization of KCa3.1. B) GST-TUBE pulldown assay, followed by IB using α-streptavidin Ab, suggests that KCa3.1 may be ubiquitylated at the plasma membrane in the absence of endocytosis (compare lanes 5 and 2) and that inhibition of ubiquitylation blocks endocytosis of the channel (UBEI-41 treatment). Subsequent to endocytosis, KCa3.1 becomes heavily polyubiquitinated (_T_=90 min, lane 3).
Figure 3.
KCa3.1 is deubiquitylated before delivery to lysosomes. A) BLAP-KCa3.1 was enzymatically biotinylated and streptavidin labeled; the channel was allowed to internalize for the times indicated in the absence or presence of PR-619. PR-619 treatment significantly inhibited KCa3.1 degradation (bar graph, _n_=3). *P < 0.05. B) Cells were treated and labeled as in A, and the ubiquitylation of KCa3.1 was assessed by GST-TUBE pulldown assay. A representative blot is shown, indicating that PR-619 treatment prevents channel deubiquitylation. These data demonstrate that KCa3.1 needs to be deubiquitylated for proper lysosomal degradation.
Figure 4.
DUB Chip as a tool to identify specific DUBs interacting with KCa3.1. A) BLAP-KCa3.1 was labeled with streptavidin-Alexa488 and incubated for the times indicated at 37°C. B) Cells were lysed in the presence of GST-TUBE2; the lysates were pulled down on GST beads, eluted, and hybridized on a DUB Chip (top panel). An interaction between fluorescently-tagged KCa3.1 and specific DUBs was quantified by measuring the fluorescence intensity (see Materials and Methods). DUB Chip data, expressed as relative fluorescence units (RFU), indicates an interaction between ubiquitylated KCa3.1 and both USP2 and USP8, and a weaker association with AMSH (bottom panel). C) Co-IP confirmed the interaction between KCa3.1 and USP8. Cells were cotransfected with myc-tagged KCa3.1 and either the WT or DN form of the GFP-USP8. USP8 was immunoprecipitated using either an anti-GFP Ab (lanes 1–3 and 5–6) or a nonspecific IgG (lanes 4 and 7) and subsequently IB for KCa3.1 with α-myc Ab. Total cell lysates are shown in lanes 8 and 9.
Figure 5.
USP8 overexpression alters the ubiquitylation and degradation rate of KCa3.1 A) Cells were doubly transfected with BLAP-KCa3.1 and either GFP vector alone or the WT or DN GFP-USP8. KCa3.1 was labeled at the cell surface with streptavidin-Alexa555, and cells were incubated at 37°C for 8 h. In cells overexpressing either form of USP8, KCa3.1 degradation is slowed compared with control cells, as shown by the strong intracellular signal. Moreover, KCa3.1 clearly colocalizes with DN GFP-USP8 subsequent to endocytosis (bottom panel, arrow in overlay). B) Degradation rate of membrane KCa3.1 was quantified as above. Both WT and DN GFP-USP8 caused a significant delay in KCa3.1 degradation rate (bar graph, _n_=3). *P < 0.05. C) To correlate the degradation in B with the level of KCa3.1 ubiquitylation, cells were prepared as in B and lysed in the presence of GST-TUBE2 at the indicated times, followed by pulldown on GST beads and IB using α-streptavidin Ab. WT USP8 overexpression decreases ubiquitylation of KCa3.1, while DN USP8 prevents deubiquitylation, as compared with control cells.
Figure 6.
Knockdown of USP8 prevents KCa3.1 deubiquitylation and channel degradation. A) Cells stably expressing BLAP-KCa3.1 were transfected with siRNA control or USP8-specific siRNA. Degradation rate of the channel was significantly inhibited in USP8-depleted cells (bar graph, _n_=3). *P < 0.05. B) Cells were treated and labeled as in A. Ubiquitylation of KCa3.1 was assessed by GST-TUBE pulldown assay. A representative blot is shown, indicating that knockdown of the endogenous USP8 impairs deubiquitylation of internalized KCa3.1.
Similar articles
- ESCRT-dependent targeting of plasma membrane localized KCa3.1 to the lysosomes.
Balut CM, Gao Y, Murray SA, Thibodeau PH, Devor DC. Balut CM, et al. Am J Physiol Cell Physiol. 2010 Nov;299(5):C1015-27. doi: 10.1152/ajpcell.00120.2010. Epub 2010 Aug 18. Am J Physiol Cell Physiol. 2010. PMID: 20720181 Free PMC article. - Ubiquitin-specific peptidase 8 (USP8) regulates endosomal trafficking of the epithelial Na+ channel.
Zhou R, Tomkovicz VR, Butler PL, Ochoa LA, Peterson ZJ, Snyder PM. Zhou R, et al. J Biol Chem. 2013 Feb 22;288(8):5389-97. doi: 10.1074/jbc.M112.425272. Epub 2013 Jan 7. J Biol Chem. 2013. PMID: 23297398 Free PMC article. - Anterograde trafficking of KCa3.1 in polarized epithelia is Rab1- and Rab8-dependent and recycling endosome-independent.
Bertuccio CA, Lee SL, Wu G, Butterworth MB, Hamilton KL, Devor DC. Bertuccio CA, et al. PLoS One. 2014 Mar 14;9(3):e92013. doi: 10.1371/journal.pone.0092013. eCollection 2014. PLoS One. 2014. PMID: 24632741 Free PMC article. - Regulation of endocytic sorting by ESCRT-DUB-mediated deubiquitination.
Wright MH, Berlin I, Nash PD. Wright MH, et al. Cell Biochem Biophys. 2011 Jun;60(1-2):39-46. doi: 10.1007/s12013-011-9181-9. Cell Biochem Biophys. 2011. PMID: 21448666 Review. - [Link between endosomal membrane traffic and cytokinesis].
Mukai A, Komada M. Mukai A, et al. Tanpakushitsu Kakusan Koso. 2008 Dec;53(16 Suppl):2094-8. Tanpakushitsu Kakusan Koso. 2008. PMID: 21038591 Review. Japanese. No abstract available.
Cited by
- The E3 ubiquitin ligase ARIH1 protects against genotoxic stress by initiating a 4EHP-mediated mRNA translation arrest.
von Stechow L, Typas D, Carreras Puigvert J, Oort L, Siddappa R, Pines A, Vrieling H, van de Water B, Mullenders LH, Danen EH. von Stechow L, et al. Mol Cell Biol. 2015 Apr;35(7):1254-68. doi: 10.1128/MCB.01152-14. Epub 2015 Jan 26. Mol Cell Biol. 2015. PMID: 25624349 Free PMC article. - The Role of Deubiquitinating Enzymes in the Various Forms of Autophagy.
Csizmadia T, Lőw P. Csizmadia T, et al. Int J Mol Sci. 2020 Jun 12;21(12):4196. doi: 10.3390/ijms21124196. Int J Mol Sci. 2020. PMID: 32545524 Free PMC article. Review. - Modulation of Retrograde Trafficking of KCa3.1 in a Polarized Epithelium.
Lee BS, Devor DC, Hamilton KL. Lee BS, et al. Front Physiol. 2017 Jul 18;8:489. doi: 10.3389/fphys.2017.00489. eCollection 2017. Front Physiol. 2017. PMID: 28769813 Free PMC article. - Plasma membrane insertion of KCa2.3 (SK3) is dependent upon the SNARE proteins, syntaxin-4 and SNAP23.
Bertuccio CA, Wang TT, Hamilton KL, Rodriguez-Gil DJ, Condliffe SB, Devor DC. Bertuccio CA, et al. PLoS One. 2018 May 16;13(5):e0196717. doi: 10.1371/journal.pone.0196717. eCollection 2018. PLoS One. 2018. PMID: 29768434 Free PMC article. - The exocyst complex is required for the trafficking and delivery of KCa3.1 to the basolateral membrane of polarized epithelia.
Logue MJE, Farquhar RE, Eckhoff-Björngard Y, Cheung TT, Devor DC, McDonald FJ, Hamilton KL. Logue MJE, et al. Am J Physiol Cell Physiol. 2023 Jun 1;324(6):C1249-C1262. doi: 10.1152/ajpcell.00374.2022. Epub 2023 May 1. Am J Physiol Cell Physiol. 2023. PMID: 37125772 Free PMC article.
References
- Wulff H., Kolski-Andreaco A., Sankaranarayanan A., Sabatier J. M., Shakkottai V. (2007) Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr. Med. Chem. 14, 1437–1457 - PubMed
- Jones H. M., Hamilton K. L., Devor D. C. (2005) Role of an S4–S5 linker lysine in the trafficking of the Ca2+ -activated K+ channels IK1 and SK3. J. Biol. Chem. 280, 37257–37265 - PubMed
- Syme C. A., Hamilton K. L., Jones H. M., Gerlach A. C., Giltinan L., Papworth G. D., Watkins S. C., Bradbury N. A., Devor D. C. (2003) Trafficking of the Ca2+-activated K+ channel, hIK1, is dependent upon a C-terminal leucine zipper. J. Biol. Chem. 278, 8476–8486 - PubMed
- Raiborg C., Stenmark H. (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous