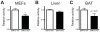Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity - PubMed (original) (raw)
Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity
Lydia W S Finley et al. PLoS One. 2011.
Abstract
Background: Sirtuins (SIRT1-7) are a family of NAD-dependent deacetylases and/or ADP-ribosyltransferases that are involved in metabolism, stress responses and longevity. SIRT3 is localized to mitochondria, where it deacetylates and activates a number of enzymes involved in fuel oxidation and energy production.
Methodology/principal findings: In this study, we performed a proteomic screen to identify SIRT3 interacting proteins and identified several subunits of complex II and V of the electron transport chain. Two subunits of complex II (also known as succinate dehydrogenase, or SDH), SDHA and SDHB, interacted specifically with SIRT3. Using mass spectrometry, we identified 13 acetylation sites on SDHA, including six novel acetylated residues. SDHA is hyperacetylated in SIRT3 KO mice and SIRT3 directly deacetylates SDHA in a NAD-dependent manner. Finally, we found that SIRT3 regulates SDH activity both in cells and in murine brown adipose tissue.
Conclusions/significance: Our study identifies SDHA as a binding partner and substrate for SIRT3 deacetylase activity. SIRT3 loss results in decreased SDH enzyme activity, suggesting that SIRT3 may be an important physiological regulator of SDH activity.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. SIRT3 interacts with subunits of complex II and complex V.
FLAG-IPs were performed on HEK293T cells transiently expressing FLAG-tagged sirtuins (T1-7). (A) SIRT1-7 IPs were immunoblotted with antibodies against SDHA, OSCP and FLAG. (B) SIRT3-5 IPs were immunoblotted with an antibody cocktail recognizing the SDHA and SDHB subunits of complex II and the F1α subunit of complex V (top) or a cocktail containing antibodies against representative subunits of complexes I–V (middle). SDHB is the subunit recognized by the complex II antibody.
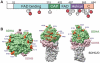
Figure 2. SDHA is acetylated at 13 lysine residues.
(A) Schematic of SDHA summarizing the four domains (FAD-binding, capping, helical and C-terminal) with 13 identified acetylated residues shown in red (novel) or white (previously identified). (B) The 13 acetylated lysines were mapped on to the corresponding residues of the crystal structure of avian complex II.
Figure 3. SIRT3 deacetylates SDHA.
Complex II (A) and acetylated proteins (B) were immunoprecipitated from liver mitochondria isolated from SIRT3 WT and KO mice. IPs were immunoblotted with antibodies against acetyl-lysine (AcK), SDHA, SDHB and GDH. (C) Complex II immunoprecipitated from mouse liver mitochondria was incubated with recombinant SIRT3 or catalytically inactive SIRT3 (SIRT3 H248Y) in the presence or absence of NAD and NAM, a sirtuin inhibitor. After deacetylation, IPs were immunoblotted using antibodies against acetylated proteins, SDHA and SIRT3. In all panels, antibodies against GFP were used as negative controls.
Figure 4. SIRT3 regulates SDH activity.
Succinate dehydrogenase activity was measured in (A) SIRT3 WT and KO MEF extracts (n = 4), (B) liver mitochondria (n = 3) and (C) brown adipose tissue (BAT) mitochondria (n = 6–7). SDH activity was normalized to sample protein content and expressed as a ratio of WT levels. Values are expressed as mean ±SEM. *, P<0.05.
Similar articles
- Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria.
Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Cimen H, et al. Biochemistry. 2010 Jan 19;49(2):304-11. doi: 10.1021/bi901627u. Biochemistry. 2010. PMID: 20000467 Free PMC article. - NAD+-dependent deacetylase SIRT3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10.
Yang Y, Cimen H, Han MJ, Shi T, Deng JH, Koc H, Palacios OM, Montier L, Bai Y, Tong Q, Koc EC. Yang Y, et al. J Biol Chem. 2010 Mar 5;285(10):7417-29. doi: 10.1074/jbc.M109.053421. Epub 2009 Dec 30. J Biol Chem. 2010. PMID: 20042612 Free PMC article. - SIRT3 (Sirtuin-3) Prevents Ang II (Angiotensin II)-Induced Macrophage Metabolic Switch Improving Perivascular Adipose Tissue Function.
Wei (魏彤) T, Gao (高晶) J, Huang (黄程淋) C, Song (宋蓓) B, Sun (孙孟炜) M, Shen (沈伟利) W. Wei (魏彤) T, et al. Arterioscler Thromb Vasc Biol. 2021 Feb;41(2):714-730. doi: 10.1161/ATVBAHA.120.315337. Epub 2020 Dec 17. Arterioscler Thromb Vasc Biol. 2021. PMID: 33327751 - SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism.
Hirschey MD, Shimazu T, Huang JY, Schwer B, Verdin E. Hirschey MD, et al. Cold Spring Harb Symp Quant Biol. 2011;76:267-77. doi: 10.1101/sqb.2011.76.010850. Epub 2011 Nov 23. Cold Spring Harb Symp Quant Biol. 2011. PMID: 22114326 Review. - Mitochondrial protein acetylation regulates metabolism.
Anderson KA, Hirschey MD. Anderson KA, et al. Essays Biochem. 2012;52:23-35. doi: 10.1042/bse0520023. Essays Biochem. 2012. PMID: 22708561 Free PMC article. Review.
Cited by
- Ginsenoside Rg1 protects against Sca-1+ HSC/HPC cell aging by regulating the SIRT1-FOXO3 and SIRT3-SOD2 signaling pathways in a γ-ray irradiation-induced aging mice model.
Tang YL, Zhou Y, Wang YP, He YH, Ding JC, Li Y, Wang CL. Tang YL, et al. Exp Ther Med. 2020 Aug;20(2):1245-1252. doi: 10.3892/etm.2020.8810. Epub 2020 May 28. Exp Ther Med. 2020. PMID: 32765665 Free PMC article. - SIRT3 deficiency decreases oxidative metabolism capacity but increases lifespan in male mice under caloric restriction.
Dhillon RS, Qin YA, van Ginkel PR, Fu VX, Vann JM, Lawton AJ, Green CL, Manchado-Gobatto FB, Gobatto CA, Lamming DW, Prolla TA, Denu JM. Dhillon RS, et al. Aging Cell. 2022 Dec;21(12):e13721. doi: 10.1111/acel.13721. Epub 2022 Oct 5. Aging Cell. 2022. PMID: 36199173 Free PMC article. - Impairment of electron transport chain and induction of apoptosis by chrysin nanoparticles targeting succinate-ubiquinone oxidoreductase in pancreatic and lung cancer cells.
Ragab EM, El Gamal DM, Mohamed TM, Khamis AA. Ragab EM, et al. Genes Nutr. 2023 Mar 11;18(1):4. doi: 10.1186/s12263-023-00723-4. Genes Nutr. 2023. PMID: 36906524 Free PMC article. - Multiomics reveals multilevel control of renal and systemic metabolism by the renal tubular circadian clock.
Bignon Y, Wigger L, Ansermet C, Weger BD, Lagarrigue S, Centeno G, Durussel F, Götz L, Ibberson M, Pradervand S, Quadroni M, Weger M, Amati F, Gachon F, Firsov D. Bignon Y, et al. J Clin Invest. 2023 Apr 17;133(8):e167133. doi: 10.1172/JCI167133. J Clin Invest. 2023. PMID: 36862511 Free PMC article. - Mitochondrial dysfunction in pathophysiology of heart failure.
Zhou B, Tian R. Zhou B, et al. J Clin Invest. 2018 Aug 31;128(9):3716-3726. doi: 10.1172/JCI120849. Epub 2018 Aug 20. J Clin Invest. 2018. PMID: 30124471 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous