Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection - PubMed (original) (raw)
Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection
Elaine K Lau et al. Sci Signal. 2011.
Abstract
In comparison to endogenous ligands of seven-transmembrane receptors, which typically act as full agonists, many drugs act as partial agonists. Partial agonism is best described as a "macroscopic" property that is manifest at the level of physiological systems or cell populations; however, whether partial agonists also encode discrete regulatory information at the "microscopic" level of individual receptors is not known. Here, we addressed this question by focusing on morphine, a partial agonist drug for μ-type opioid peptide receptors (MORs), and by combining quantitative mass spectrometry with cell biological analysis to investigate the reduced efficacy of morphine, compared to that of a peptide full agonist, in promoting receptor endocytosis. We showed that these chemically distinct ligands produced a complex and qualitatively similar mixture of phosphorylated opioid receptor forms in intact cells. Quantitatively, however, the different agonists promoted disproportionate multisite phosphorylation of a specific serine and threonine motif, and we found that modification at more than one residue was essential for the efficient recruitment of the adaptor protein β-arrestin that mediated subsequent endocytosis of MORs. Thus, quantitative encoding of agonist-selective endocytosis at the level of individual opioid receptors was based on the conserved biochemical principles of multisite phosphorylation and threshold detection.
Figures
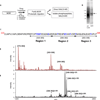
Figure 1. Overview of MS Analyses
(A) Experimental scheme for processing MOR for analyses by mass spectrometry (MS) with or without phospho-enrichment via immobilized metal ion affinity chromatography (IMAC). HEK293 cells expressing WT MOR were treated with 10 µM morphine, 10 µM DAMGO, or left untreated (No Drug) for 20 minutes at 37C. (B) Receptors were purified from cell pellets as described in Materials and Methods, subjected to SDS-PAGE, and detected with SYPRO Ruby total protein stain. (C) Sequence of MOR C-Terminal tail. Highlighted in blue are the potential phosphorylation sites. (D) MS of MOR tryptic peptides. Full coverage of Ser and Thr residues in the C-tail of MOR are observed unphosphorylated: [349–365] m/z= 1967.92 (calc m/z= 1967.92); [372–382] m/z= 1226.58 (calc m/z= 1226.58); [383–398] m/z= 1776.88 (calc m/z= 1776.88). (E) MS of phosphopeptides after enrichment using IMAC procedure, reveals MOR phosphorylation for Region 1: [349–365]+1Pi m/z= 2047.86 (calc m/z= 2047.88); [349–365]+2Pi m/z= 2127.86 (calc m/z= 2127.85) and Region 2: [368–382]+1Pi m/z= 1805.79 (calc m/z= 1805.79); [366–382]+1Pi m/z= 2074.97(calc m/z= 2074.98); [366–382]+2Pi m/z=2154.93 (calc m/z= 2154.95); [366–382]+3Pi m/z= 2234.91(calc m/z= 2234.91). Region 3 is not observed phosphorylated.
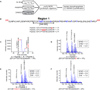
Figure 2. LC-ESI MS of phosphorylated MOR region 1 under three different conditions after 20 minutes of agonist exposure
(A) Schematic diagram of experiment. Use of stable isotope labeling of MOR allows all three conditions (ND= No Drug with 12C, 14N-Arg; M= +Morphine with 13C, 14N-Arg; D= +DAMGO with 13C,15N-Arg) to be purified, handled and analyzed simultaneously. Adjusted isotope ratios were calculated by summing signal count areas for 2–3 mass isotope peaks, adjusted for isotope overlap, and calibrated using the internal MOR reference peptide multipliers (1.1x for (M); 1.4x for (D)). (B) MOR c-terminal tail peptide sequence, specifying region 1 as the phosphorylated peptides of EFCIPTSSTIEQQNSAR. (C) Using LC-ESI MS, we observed two separate elutions of singly phosphorylated EFCIPTSSTIEQQNSAR, at 29.3 min and 30.5 min. Peptide 1 with phosphorylation site at Ser363 eluted at 29.3 min. Peptide 2 with phosphorylation localized within the 354TSST357 region eluted at 30.5 min. (D) At elution time 29.3 min, monoisotopic signals were observed at m/z= 1024.5, 1027.5, and 1029.5 corresponding to [M+2H]+2 of peptide 1 under the three agonist conditions ND, M, and D, respectively. Adjusted isotope ratios calculated for peptide 1 includeM/ND= 1.1, D/ND= 1.0 and D/M=0.9. (E) At elution time 30.5 min, monoisotopic signals were observed at m/z= 1024.4, 1027.5, and 1029.5 corresponding to [M+2H]+2 of peptide 2 under the three agonist conditions ND, M, and D, respectively. Adjusted isotope ratios calculated for peptide 2 include M/ND= 4.6, D/ND= 9.3, and D/M= 2.0. (F) At elution time 31 min, peptide 3 elutes as a single peak on the LC with monoisotopic signals observed at m/z= 1064.4, 1067.4, and 1069.4 corresponding to [M+2H]+2 of peptide 3 under the three agonist conditions ND, M, D, respectively. Adjusted isotope ratios calculated for peptide 3 include M/ND= 2.8, D/ND= 2.9, and D/M= 1.0.
Figure 3. LC-ESI MS of phosphorylated MOR region 2 under three different conditions after 20 minutes of agonist exposure
(A) Using LC-ESI MS, we observed phosphorylated region 2 in two different overlapping sequences, QNTREHPSTANTVDR and EHPSTANTVDR. Adjusted isotope ratios were calculated by summing signal count areas for 2–3 mass isotope peaks, adjusted for isotope overlap, and calibrated using the internal MOR reference peptide multipliers (1.1x for (M); 1.4x for (D)). (B) Peptide 4 with phosphorylation site at Thr370 eluted at 15.9 min. Monoisotopic signals were observed at m/z= 602.6, 606.6, and 609.3 corresponding to [M+3H]+3 of peptide 4 under the three agonist conditions ND, M, D, respectively. Adjusted isotope ratios calculated for peptide 4 include M/ND= 1.4, D/ND=2.5 and D/M= 1.8. (C) At elution time 15.9 min, monoisotopic signals were observed at m/z= 653.8, 656.8, and 658.8 corresponding to [M+2H]+2 of peptide 5 under the three agonist conditions ND, M, D, respectively. Similar to peptide 4, adjusted isotope ratios calculated for peptide 5 include M/ND= 1.6, D/ND= 2.7,and D/M= 1.7. (D) At elution time 16.5 min, monoisotopic signals were observed at m/z= 629.3, 633.3 and 635.9 corresponding to [M+3H]+3 of peptide 6 under the three agonist conditions ND, M, D, respectively. Adjusted isotope ratios calculated for peptide 6 include M/ND= 3.5, D/ND= 9.2, and D/M= 2.6. (E) At elution time 16.5min, monoisotopic signals were observed at m/z=696.8, and 698.8 corresponding to [M+2H]+2 of peptide 7 under the two agonist conditions M and D, respectively. No signal above noise was observed for ND treatment of peptide 7 at m/z= 692.8 and therefore the adjusted isotope ratio calculated was D/M= 4.3.
Figure 4. Independent LC-ESI MS analysis of phosphorylated MOR region 2 after 180 minutes of agonist exposure
An independent series of peptides spanning region 2, generated by variation of trypsin cleavage and representing single (+1Pi), double (+2Pi) and triple (+3Pi) receptor phosphorylation in this region. Adjusted isotope ratios were calculated by summing signal count areas for 2–3 mass isotope peaks, adjusted for isotope overlap, and calibrated using the internal MOR reference peptide multipliers (1.5x for (M); 3.3x for (D)). (A) Singly phosphorylated (+1Pi) peptide eluted at 13.4 min. Monoisotopic signals were observed at m/z= 692.3, 698.3, and 702.3 corresponding to [M+3H]+3 of peptide 8 under the three agonist conditions ND, M, D, respectively. Adjusted isotope ratios (D/M value is also indicated in the figure): M/ND= 1.1; D/ND= 2.9; and D/M= 2.7 (B) Doubly phosphorylated (+2Pi) peptide eluted at 14.4 min. Monoisotopic signals were observed at m/z= 725.0, 729.0, corresponding to [M+3H]+3 of peptide 9 under the two agonist conditions M and D, respectively. No signal above noise was observed for ND at m/z= 718.9. Adjusted isotope ratio: D/M= 4.5. (C) Triply phosphorylated (+3Pi) peptide eluted at 14.6 min. Monoisotopic signals were observed at m/z= 751.6 and 755.6 corresponding to [M+3H]+3 of peptide 10 under the three agonist conditions M and D, respectively. No signal above noise was observed for ND at m/z= 745.6. Adjusted isotope ratio: D/M= 14.8.
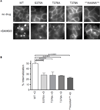
Figure 5. Effects of 354AAAA357 and 375AAANA379 mutations on regulated endocytosis of MORs
(A) HEK293 cells stably transfected with the indicated Flag epitope-tagged MOR constructs were incubated in the absence of agonist (ND) or exposed to 10 µM DAMGO (D) or morphine (M) for 20 minutes at 37°C. Cells were then chemically fixed, and receptors detected and localized with Alexa 488-conjugated M1 antibody as described in Materials and Methods. (B) Quantification of endocytic effects by fluorescence flow cytometry, based on agonist-induced reduction in surface receptor immunoreactivity as described in Materials and Methods. In each experiment, and for each condition, triplicate analyses were carried out using 10,000 cells / analysis. For each receptor construct, at least two independently isolated cell clones were tested. Bars represent averaged internalization values across independent experiments (WT n=10; 354AAAA357 n=4; 375AAANA379 n=4 experiments). Error bars represent S.E.M. and p-values were calculated using Student’s t-test.
Figure 6. Fine structure analysis of endocytic activity conferred by the 375STANT379 motif
(A) HEK293 cells stably transfected with the indicated Flag epitope-tagged MOR constructs were analyzed as in Figure 5. (B) Flow cytometric quantification of mutational effects on DAMGO-induced endocytosis (WT n=10; S375A n=6; T376A n=4; T379A n=4; 375AAANA379 n=4 experiments; each condition in triplicate.). Values are expressed as mean +/− S.E.M., with p-values calculated by Student’s t-test.
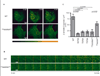
Figure 7. Contribution of the 375STANT379 motif to receptor-mediated recruitment of β-arrestin to clathrin-coated pits
(A) HEK293 cells co-expressing β-arrestin-2 (arrestin 3)-GFP and the indicated receptor construct were imaged live by TIR-FM. Representative frames from 0, 2 min, or 4 min after 10 uM DAMGO addition are shown. For clarity of presentation, fluorescence intensity values were pseudo-colored using the gradient shown. (B) Magnified frames 12 s apart from the representative areas delineated by the squares in panel A are shown. The time course illustrates the appearance of arrestin 3-GFP puncta from time of agonist addition through a plateau of maximum fluorescence intensity. (C) Quantification of arrestin localization in HEK293 cells co-expressing arrestin 3-GFP and the indicated receptor construct, and fixed 7 min after bath application of 10 uM DAMGO. Values for each condition (expressed as mean +/− S.E.M.) represent number of puncta visualized per unit surface area in multiple cells (WT n=27 cells; S375A n=20; T376A n=24; T379A n=16; 375AAANA379 n=50; 354AAAA357 n=17; p-values were calculated by Student’s t-test and are only indicated for p<0.05).
Similar articles
- Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons.
Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Haberstock-Debic H, et al. J Neurosci. 2005 Aug 24;25(34):7847-57. doi: 10.1523/JNEUROSCI.5045-04.2005. J Neurosci. 2005. PMID: 16120787 Free PMC article. - RGS14 prevents morphine from internalizing Mu-opioid receptors in periaqueductal gray neurons.
Rodríguez-Muñoz M, de la Torre-Madrid E, Gaitán G, Sánchez-Blázquez P, Garzón J. Rodríguez-Muñoz M, et al. Cell Signal. 2007 Dec;19(12):2558-71. doi: 10.1016/j.cellsig.2007.08.003. Epub 2007 Aug 15. Cell Signal. 2007. PMID: 17825524 - Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor.
Beyer A, Koch T, Schröder H, Schulz S, Höllt V. Beyer A, et al. J Neurochem. 2004 May;89(3):553-60. doi: 10.1111/j.1471-4159.2004.02340.x. J Neurochem. 2004. PMID: 15086512 - Morphine induces endocytosis of neuronal mu-opioid receptors through the sustained transfer of Galpha subunits to RGSZ2 proteins.
Rodríguez-Muñoz M, de la Torre-Madrid E, Sánchez-Blázquez P, Garzón J. Rodríguez-Muñoz M, et al. Mol Pain. 2007 Jul 17;3:19. doi: 10.1186/1744-8069-3-19. Mol Pain. 2007. PMID: 17634133 Free PMC article. - Opioid regulation of mu receptor internalisation: relevance to the development of tolerance and dependence.
Lopez-Gimenez JF, Milligan G. Lopez-Gimenez JF, et al. CNS Neurol Disord Drug Targets. 2010 Nov;9(5):616-26. doi: 10.2174/187152710793361522. CNS Neurol Disord Drug Targets. 2010. PMID: 20632966 Review.
Cited by
- Efficacy and ligand bias at the μ-opioid receptor.
Kelly E. Kelly E. Br J Pharmacol. 2013 Aug;169(7):1430-46. doi: 10.1111/bph.12222. Br J Pharmacol. 2013. PMID: 23646826 Free PMC article. Review. - Exploring Morphine-Triggered PKC-Targets and Their Interaction with Signaling Pathways Leading to Pain via TrkA.
Pena DA, Duarte ML, Pramio DT, Devi LA, Schechtman D. Pena DA, et al. Proteomes. 2018 Oct 6;6(4):39. doi: 10.3390/proteomes6040039. Proteomes. 2018. PMID: 30301203 Free PMC article. Review. - Recent Progress in Opioid Research from an Electrophysiological Perspective.
Birdsong WT, Williams JT. Birdsong WT, et al. Mol Pharmacol. 2020 Oct;98(4):401-409. doi: 10.1124/mol.119.119040. Epub 2020 Mar 20. Mol Pharmacol. 2020. PMID: 32198208 Free PMC article. Review. - Different mechanisms of homologous and heterologous μ-opioid receptor phosphorylation.
Mann A, Illing S, Miess E, Schulz S. Mann A, et al. Br J Pharmacol. 2015 Jan;172(2):311-6. doi: 10.1111/bph.12627. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24517854 Free PMC article. Review. - Dissecting the roles of GRK2 and GRK3 in μ-opioid receptor internalization and β-arrestin2 recruitment using CRISPR/Cas9-edited HEK293 cells.
Møller TC, Pedersen MF, van Senten JR, Seiersen SD, Mathiesen JM, Bouvier M, Bräuner-Osborne H. Møller TC, et al. Sci Rep. 2020 Oct 15;10(1):17395. doi: 10.1038/s41598-020-73674-0. Sci Rep. 2020. PMID: 33060647 Free PMC article.
References
- Bradbury AF, Smyth DG, Snell CR. Biosynthetic origin and receptor conformation of methionine enkephalin. Nature. 1976 Mar 11;260:165. - PubMed
- Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene [see comments] Nature. 1996;383:819. - PubMed
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003 May 23;278:18776. - PubMed
- Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007 Oct;17:556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DA010154/DA/NIDA NIH HHS/United States
- F32-DA020972-01/DA/NIDA NIH HHS/United States
- R37 DA010711/DA/NIDA NIH HHS/United States
- DA012864/DA/NIDA NIH HHS/United States
- F32 DA020972/DA/NIDA NIH HHS/United States
- R01 DA006511/DA/NIDA NIH HHS/United States
- R01 DA012864/DA/NIDA NIH HHS/United States
- P41 RR001614/RR/NCRR NIH HHS/United States
- DA06511/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials