Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132 - PubMed (original) (raw)
Comparative Study
. 2011 Sep 30;109(8):894-906.
doi: 10.1161/CIRCRESAHA.111.251546. Epub 2011 Aug 25.
Federica Riu, Kathryn Mitchell, Miriam Gubernator, Paola Campagnolo, Yuxin Cui, Orazio Fortunato, Elisa Avolio, Daniela Cesselli, Antonio Paolo Beltrami, Gianni Angelini, Costanza Emanueli, Paolo Madeddu
Affiliations
- PMID: 21868695
- PMCID: PMC3623091
- DOI: 10.1161/CIRCRESAHA.111.251546
Comparative Study
Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132
Rajesh Katare et al. Circ Res. 2011.
Abstract
Rationale: Pericytes are key regulators of vascular maturation, but their value for cardiac repair remains unknown.
Objective: We investigated the therapeutic activity and mechanistic targets of saphenous vein-derived pericyte progenitor cells (SVPs) in a mouse myocardial infarction (MI) model.
Methods and results: SVPs have a low immunogenic profile and are resistant to hypoxia/starvation (H/S). Transplantation of SVPs into the peri-infarct zone of immunodeficient CD1/Foxn-1(nu/nu) or immunocompetent CD1 mice attenuated left ventricular dilatation and improved ejection fraction compared to vehicle. Moreover, SVPs reduced myocardial scar, cardiomyocyte apoptosis and interstitial fibrosis, improved myocardial blood flow and neovascularization, and attenuated vascular permeability. SVPs secrete vascular endothelial growth factor A, angiopoietin-1, and chemokines and induce an endogenous angiocrine response by the host, through recruitment of vascular endothelial growth factor B expressing monocytes. The association of donor- and recipient-derived stimuli activates the proangiogenic and prosurvival Akt/eNOS/Bcl-2 signaling pathway. Moreover, microRNA-132 (miR-132) was constitutively expressed and secreted by SVPs and remarkably upregulated, together with its transcriptional activator cyclic AMP response element-binding protein, on stimulation by H/S or vascular endothelial growth factor B. We next investigated if SVP-secreted miR-132 acts as a paracrine activator of cardiac healing. In vitro studies showed that SVP conditioned medium stimulates endothelial tube formation and reduces myofibroblast differentiation, through inhibition of Ras-GTPase activating protein and methyl-CpG-binding protein 2, which are validated miR-132 targets. Furthermore, miR-132 inhibition by antimiR-132 decreased SVP capacity to improve contractility, reparative angiogenesis, and interstitial fibrosis in infarcted hearts.
Conclusion: SVP transplantation produces long-term improvement of cardiac function through a novel paracrine mechanism involving the secretion of miR-132 and inhibition of its target genes.
Figures
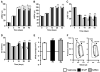
Figure 1. SVP transplantation prevents late LV dysfunction in immunocompetent mice
A–E, Bar graphs showing hemodynamic parameters. F, Representative pressure/volume loops. Data are means±SE (n=13 mice per group). **P<0.01 vs vehicle-treated; §§P<0.01 vs corresponding-treatment group at 14 days post-MI; ɸP<0.05 vs SVP-transplanted.
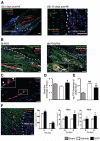
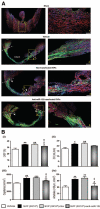
Figure 2. Graft localization and promotion of angiogenesis
A–C, Representative confocal images showing the localization of Dil-labeled SVPs (red) at 5 (Ai) and 42 days (Aii) post-MI. Dil-labeled SVPs, positive for NG2 (white, Bi) and PDGFR_β_ (white, Bii), are juxta-posed to ECs identified by intravenously-injected isolectin (green). Some SVPs are positive for PCNA (pale blue, arrowheads, C). Nuclei stained blue by DAPI. Scale bars are 50 _μ_m. D, E, Bar graphs showing LV myocardial blood flow (D) and vascular permeability (E). F, Representative fluorescent image and bar graph showing the density of capillaries, assessed by counting small-size (<10 _μ_m in diameter) isolectin-positive structures, and arterioles, assessed by counting _α_-smooth muscle actin-positive structures. Scale bars are 50 _μ_m. Dotted lines delineate the infarct zone. Data are means±SE (n=6 in each group). ##P<0.01 and ###P<0.001 vs sham; *P<0.05 and **P<0.01 vs vehicle.
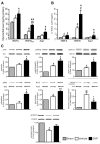
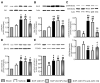
Figure 3. Activation of angiogenic factors and survival kinases
A, B, Bar graphs showing the protein (A) and mRNA (B) levels of murine GFs in myocardium at 14 days post-MI. C, Representative western blotting and bar graphs showing myocardial activation of survival signaling pathway at 14 days post-MI. Data are means±SE (n=6 mice per group). #P<0.05 and ##P<0.01 vs sham; *P<0.05 and **P<0.01 vs vehicle.
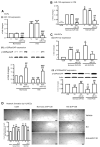
Figure 4. SVPs exert paracrine effects through miR-132
A, Bar graphs showing induction of miR-132 (Ai) and inhibition of its target p120RasGAP (Aii) in SVPs exposed to H/S. AntimiR-132 transfection (50nmol/L) suppressed miR-132 expression while inducing p120RasGAP expression (Aii). Control SVPs were transfected with scrambled sequence (Scr, 50nmol/L), or exposed to transfectionvehicle (V). B. Levels of miR-132 in SVP-CM. Data are means±SE from experiments performed in quadruplicates. *P<0.05, **P<0.01, and ***P<0.001 vs corresponding group under normoxia; §§P<0.01 and §§§P<0.001 vs vehicle; ##P<0.01 and ###P<0.001 vs Scr. C, Bar graphs showing miR-132 levels (Ci) and p120RasGAP protein expression (Cii) in HUVECs exposed to SVP-CM collected under different conditions described above. D, Representative images and bar graphs showing the ability of SVP-CM to enhance HUVEC network formation and abrogation of this effect by CM of antimiR-132-transfected SVPs. HUVECs were cultured on matrigel with SVP-CM for 24 hours. Data are means±SE of experiments performed in quadruplicates. ɸP<0.05; ɸɸP<0.01 vs unconditioned medium (UCM); ɸɸɸP<0.001; §§P<0.01 vs corresponding group under normoxic SVP-CM; ##P<0.01 and ###P<0.001 vs Scr.
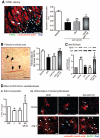
Figure 5. A, Immunofluorescence confocal microscopy images showing the levels of miR-132 target gene p120RasGAP in myocardium
Arrowheads indicate the site of SVP injection, dotted lines delimitate the infarct area and magnification panel of boxed area is shown in right panel. B, Bar graphs showing hemodynamic data (i and ii) and vascular profile (iii and iv) at 14 days post-MI. Data are means±SE (n=5 mice per group except for hemodynamic measurements which consisted of 6 mice). *P<0.05, **P<0.01, and ***P<0.001 vs vehicle; δ P<0.05 vs nontransfected SVPs; ɸP<0.05 vs Scrtransfected SVPs.
Figure 6. Inhibition of miR-132 attenuates SVP-induced activation of survival signaling
A–E, Representative immunoblots and bar graphs showing the expression of pAkt (A), Bcl-2 (B), pGSK3-beta (C), pErk1/2 (D), and pFOXO1 (E) at 14 days post-MI in the myocardium of mice treated with naïve, scrambled-transfected or antimiR-132-transfected SVPs. Data are means±SE (n=5 mice per group). #P<0.05 and ##P<0.01 vs sham; *P<0.05 and **P<0.01 vs vehicle; δP<0.05 and δδP<0.01 vs nontransfected SVPs; ɸP<0.05 and ɸɸP<0.01 vs SCr-transfected SVPs.
Figure 7. SVP transplantation attenuates cardiomyocyte apoptosis and interstitial fibrosis
A, Representative immunofluorescence image and bar graph showing abundance of apoptotic cardiomyocytes at 14 days post-MI in hearts injected with vehicle or transplanted with naïve, scrambled-transfected or antimiR-132-transfected SVPs. Data are means±SE (n=5 mice per group). *P<0.05 and **P<0.01 vs vehicle. B, Representative image and bar graph showing fibrosis in the spared myocardium. C, Representative immunoblots and bar graph showing the expression of MeCP2 in myocardium (n=5 mice per group). ##P<0.01 vs Sham; **P<0.01 vs Vehicle; δP<0.05 vs nontransfected SVPs; ɸP<0.05 vs SCrtransfected SVPs. D, Bar graph (i) and immunocytochemical images (ii) showing the effect of SVP-CM on BrdU incorporation (i) and differentiation of murine cardiac fibroblasts into myofibroblasts (ii). Cardiac fibroblasts were treated with Angiotensin II (Ang II, 100 nmol/L) for 24 hours to induce differentiation into myofibroblasts in the presence or absence of SVP-CM. Differentiated myofibroblasts are identified by positive staining for _α_-smooth muscle actin and MeCP2 (arrow head). Data are means±SE of experiments performed in quadruplicate. ɸɸP<0.01 vs unconditioned medium (UCM); **P<0.01 vs vehicle (V); ##P<0.01 vs scrambled sequence (Scr). Scale bars are 50 _μ_m.
Figure 8. Cartoon image showing the mechanism of SVP induced protection in the myocardium post-MI
Similar articles
- Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair.
Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, Oikawa A, Rodriguez-Arabaolaza I, Dang Z, Mitchell K, Reni C, Alvino VV, Rowlinson J, Livi U, Cesselli D, Angelini G, Emanueli C, Beltrami AP, Madeddu P. Avolio E, et al. Circ Res. 2015 May 8;116(10):e81-94. doi: 10.1161/CIRCRESAHA.115.306146. Epub 2015 Mar 23. Circ Res. 2015. PMID: 25801898 - Human pericytes for ischemic heart repair.
Chen CW, Okada M, Proto JD, Gao X, Sekiya N, Beckman SA, Corselli M, Crisan M, Saparov A, Tobita K, Péault B, Huard J. Chen CW, et al. Stem Cells. 2013 Feb;31(2):305-16. doi: 10.1002/stem.1285. Stem Cells. 2013. PMID: 23165704 Free PMC article. - Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential.
Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Kränkel N, Katare R, Angelini G, Emanueli C, Madeddu P. Campagnolo P, et al. Circulation. 2010 Apr 20;121(15):1735-45. doi: 10.1161/CIRCULATIONAHA.109.899252. Epub 2010 Apr 5. Circulation. 2010. PMID: 20368523 Free PMC article. - Discovering cardiac pericyte biology: From physiopathological mechanisms to potential therapeutic applications in ischemic heart disease.
Avolio E, Madeddu P. Avolio E, et al. Vascul Pharmacol. 2016 Nov;86:53-63. doi: 10.1016/j.vph.2016.05.009. Epub 2016 Jun 5. Vascul Pharmacol. 2016. PMID: 27268036 Review. - New Insights into the Reparative Angiogenesis after Myocardial Infarction.
Martín-Bórnez M, Falcón D, Morrugares R, Siegfried G, Khatib AM, Rosado JA, Galeano-Otero I, Smani T. Martín-Bórnez M, et al. Int J Mol Sci. 2023 Aug 1;24(15):12298. doi: 10.3390/ijms241512298. Int J Mol Sci. 2023. PMID: 37569674 Free PMC article. Review.
Cited by
- Loss of miR-132/212 Has No Long-Term Beneficial Effect on Cardiac Function After Permanent Coronary Occlusion in Mice.
Lei Z, Fang J, Deddens JC, Metz CHG, van Eeuwijk ECM, El Azzouzi H, Doevendans PA, Sluijter JPG. Lei Z, et al. Front Physiol. 2020 Jun 16;11:590. doi: 10.3389/fphys.2020.00590. eCollection 2020. Front Physiol. 2020. PMID: 32612537 Free PMC article. - A brief primer on microRNAs and their roles in angiogenesis.
Anand S. Anand S. Vasc Cell. 2013 Jan 16;5(1):2. doi: 10.1186/2045-824X-5-2. Vasc Cell. 2013. PMID: 23324117 Free PMC article. - MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia.
Spinetti G, Fortunato O, Caporali A, Shantikumar S, Marchetti M, Meloni M, Descamps B, Floris I, Sangalli E, Vono R, Faglia E, Specchia C, Pintus G, Madeddu P, Emanueli C. Spinetti G, et al. Circ Res. 2013 Jan 18;112(2):335-46. doi: 10.1161/CIRCRESAHA.111.300418. Epub 2012 Dec 11. Circ Res. 2013. PMID: 23233752 Free PMC article. Clinical Trial. - Pericytes in Vascular Development.
Payne LB, Hoque M, Houk C, Darden J, Chappell JC. Payne LB, et al. Curr Tissue Microenviron Rep. 2020 Sep;1(3):143-154. doi: 10.1007/s43152-020-00014-9. Epub 2020 Jul 2. Curr Tissue Microenviron Rep. 2020. PMID: 33748774 Free PMC article. - Myocardial cell-to-cell communication via microRNAs.
Ottaviani L, Sansonetti M, da Costa Martins PA. Ottaviani L, et al. Noncoding RNA Res. 2018 May 30;3(3):144-153. doi: 10.1016/j.ncrna.2018.05.004. eCollection 2018 Sep. Noncoding RNA Res. 2018. PMID: 30175287 Free PMC article. Review. No abstract available.
References
- Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart (British Cardiac Society) 2010;96:792–800. - PubMed
- Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–936. - PubMed
- Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, Tillmanns H, Assmus B, Dimmeler S, Zeiher AM, Hamm C. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. American Heart Journal. 2009;157:541–547. - PubMed
- Kang S, Yang YJ, Li CJ, Gao RL. Effects of intracoronary autologous bone marrow cells on left ventricular function in acute myocardial infarction: a systematic review and meta-analysis for randomized controlled trials. Coronary Artery Disease. 2008;19:327–335. - PubMed
- Dobaczewski M, Akrivakis S, Nasser K, Michael LH, Entman ML, Frangogiannis NG. Vascular mural cells in healing canine myocardial infarcts. J Histochem Cytochem. 2004;52:1019–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PG/09/086/BHF_/British Heart Foundation/United Kingdom
- PG/06/096/21325/BHF_/British Heart Foundation/United Kingdom
- FS/10/001/27959/BHF_/British Heart Foundation/United Kingdom
- MR/J015350/1/MRC_/Medical Research Council/United Kingdom
- PG/09/086/28048/BHF_/British Heart Foundation/United Kingdom
- PG/10/81/28606/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials