Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis - PubMed (original) (raw)
Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis
Claudia Günther et al. Nature. 2011.
Abstract
Dysfunction of the intestinal epithelium is believed to result in the excessive translocation of commensal bacteria into the bowel wall that drives chronic mucosal inflammation in Crohn's disease, an incurable inflammatory bowel disease in humans characterized by inflammation of the terminal ileum. In healthy individuals, the intestinal epithelium maintains a physical barrier, established by the tight contact of cells. Moreover, specialized epithelial cells such as Paneth cells and goblet cells provide innate immune defence functions by secreting mucus and antimicrobial peptides, which hamper access and survival of bacteria adjacent to the epithelium. Epithelial cell death is a hallmark of intestinal inflammation and has been discussed as a possible pathogenic mechanism driving Crohn's disease in humans. However, the regulation of epithelial cell death and its role in intestinal homeostasis remain poorly understood. Here we demonstrate a critical role for caspase-8 in regulating necroptosis of intestinal epithelial cells (IECs) and terminal ileitis. Mice with a conditional deletion of caspase-8 in the intestinal epithelium (Casp8(ΔIEC)) spontaneously developed inflammatory lesions in the terminal ileum and were highly susceptible to colitis. Casp8(ΔIEC) mice lacked Paneth cells and showed reduced numbers of goblet cells, indicating dysregulated antimicrobial immune cell functions of the intestinal epithelium. Casp8(ΔIEC) mice showed increased cell death in the Paneth cell area of small intestinal crypts. Epithelial cell death was induced by tumour necrosis factor (TNF)-α, was associated with increased expression of receptor-interacting protein 3 (Rip3; also known as Ripk3) and could be inhibited on blockade of necroptosis. Lastly, we identified high levels of RIP3 in human Paneth cells and increased necroptosis in the terminal ileum of patients with Crohn's disease, suggesting a potential role of necroptosis in the pathogenesis of this disease. Together, our data demonstrate a critical function of caspase-8 in regulating intestinal homeostasis and in protecting IECs from TNF-α-induced necroptotic cell death.
Figures
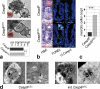
Figure 1. _Casp8_ΔIEC mice spontaneously develop ileitis and lack Paneth cells
(a) Representative endoscopic pictures and (b) H&E stained cross sections showing villous erosions in the terminal ileum of _Casp8_ΔIEC. (c) RT-PCR showing increased level of inflammatory markers in the terminal ileum of _Casp8_ΔIEC mice (6 mice per group +SEM, relative to HPRT). (d) Gene ontology analysis of genes significantly downregulated in gene chip analysis of IEC from 3 control and 3 _Casp8_ΔIEC mice. (e) Ileum cross sections stained with H&E and lysozyme for Paneth cells (inset = single crypt at higher magnification). Arrows indicate crypt bottom with Paneth cells.
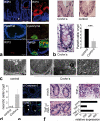
Figure 2. Increased caspase-8 independent cell death within crypts of _Casp8_ΔIEC mice
(a) Representative pictures of gut organoids. Inset = Eosin staining indicating Paneth cells. Graph: Number of Paneth cells (pc) per organoid crypt (n=24) +SEM. (b) Crypt cross sections from the small intestine of control and _Casp8_ΔIEC mice stained with H&E. TUNEL ( Inset: condensed nuclei) and caspase-3. (c) Quantification of necrotic cells per crypt + SD of control (n=9) and _Casp8_ΔIEC (n=14) mice. (d) Electron microscopic pictures of dying crypt cells in _Casp8_ΔIEC mice and inducible _Casp8_ΔIEC. Asterisks, Paneth cell granules; “n”, nuclei; “l”, crypt lumen; Arrows: mitochondrial swelling.
Figure 3. Inhibition of TNF-α induced epithelial necroptosis in _Casp8_ΔIEC mice
(a) Representative RIP3 staining colocalizing with condensed nuclei at the crypt bottom of _Casp8_ΔIEC mice. (b) Western blot for RIP3 and cleaved caspase-3 of IEC lysates isolated from TNF-α treated control and _Casp8_ΔIEC mice. Actin served as a control. (c) Representative microscopic pictures and (d) cell viability of _Casp8_ΔIEC organoids treated for 24 h with TNF-α +/- necrostatin-1. (e) Survival and (f) H&E stained small intestine cross sections of control (n=5), _Casp8_ΔIEC (mock pretreated, n=7) and _Casp8_ΔIEC (nec-1 pretreated, n=8) mice after intravenous injection of TNF-α. Asterisks show significance level relative to _Casp8_ΔIEC without nec-1. All experiment were performed at least 3 times with similar results.
Figure 4. RIP-mediated necroptosis of Paneth cells in patients with Crohn's disease
(a) Representative RIP3 immunostaining of the terminal ileum (healthy patient). TOP: RIP3 expression in human Paneth cells. BOTTOM: Colocalization of lysozyme and RIP3 in Paneth cells. (b) H&E staining of crypts in the terminal ileum. Arrows indicate crypt cells with shrunken eosinophilic cytoplasm and pyknotic nuclei. GRAPH: Number of Paneth cells (+SD) per crypt in control patients (n=7) and patients with active Crohn's disease (n=4). (c) Electron microscopy of the terminal ileum of control and Crohn's disease patient. Asterisks highlight Paneth cell granules, “n” indicates nucleus. (d) Number of crypt cells (+SD) showing organelle swelling but regular nuclei as signs of necroptosis. EM pictures of 4 patients were analyzed. (e) Representative immunofluorescence staining for TUNEL and active caspase-3 in crypts of the terminal ileum of a CD-patient. (f) H&E staining of biopsies from the small intestine of control patients stimulated in vitro with either DMSO (mock), TNF-α alone or in combination with necrostatin-1. GRAPH: Quantitative expression level of the Paneth cell marker lysozyme relative to HPRT. Data from one representative experiment out of 2 is shown. Arrow indicate Paneth cells (a,e) or mitochondrial swelling (c).
Similar articles
- Interferon Lambda Promotes Paneth Cell Death Via STAT1 Signaling in Mice and Is Increased in Inflamed Ileal Tissues of Patients With Crohn's Disease.
Günther C, Ruder B, Stolzer I, Dorner H, He GW, Chiriac MT, Aden K, Strigli A, Bittel M, Zeissig S, Rosenstiel P, Atreya R, Neurath MF, Wirtz S, Becker C. Günther C, et al. Gastroenterology. 2019 Nov;157(5):1310-1322.e13. doi: 10.1053/j.gastro.2019.07.031. Epub 2019 Jul 25. Gastroenterology. 2019. PMID: 31352002 - FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation.
Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernández-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. Welz PS, et al. Nature. 2011 Jul 31;477(7364):330-4. doi: 10.1038/nature10273. Nature. 2011. PMID: 21804564 - RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis.
Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M. Dannappel M, et al. Nature. 2014 Sep 4;513(7516):90-4. doi: 10.1038/nature13608. Epub 2014 Aug 17. Nature. 2014. PMID: 25132550 Free PMC article. - The roles and functions of Paneth cells in Crohn's disease: A critical review.
Yang E, Shen J. Yang E, et al. Cell Prolif. 2021 Jan;54(1):e12958. doi: 10.1111/cpr.12958. Epub 2020 Nov 11. Cell Prolif. 2021. PMID: 33174662 Free PMC article. Review. - Innate immune dysfunction in inflammatory bowel disease.
Gersemann M, Wehkamp J, Stange EF. Gersemann M, et al. J Intern Med. 2012 May;271(5):421-8. doi: 10.1111/j.1365-2796.2012.02515.x. Epub 2012 Feb 13. J Intern Med. 2012. PMID: 22324936 Review.
Cited by
- Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling.
Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, Xia Z, Han J. Chen W, et al. J Biol Chem. 2013 Jun 7;288(23):16247-16261. doi: 10.1074/jbc.M112.435545. Epub 2013 Apr 23. J Biol Chem. 2013. PMID: 23612963 Free PMC article. - Fueling the flames: Mammalian programmed necrosis in inflammatory diseases.
Chan FK. Chan FK. Cold Spring Harb Perspect Biol. 2012 Nov 1;4(11):a008805. doi: 10.1101/cshperspect.a008805. Cold Spring Harb Perspect Biol. 2012. PMID: 23125016 Free PMC article. Review. - Necroptosis and its role in inflammation.
Pasparakis M, Vandenabeele P. Pasparakis M, et al. Nature. 2015 Jan 15;517(7534):311-20. doi: 10.1038/nature14191. Nature. 2015. PMID: 25592536 Review. - Dysbiotic gut microbiota causes transmissible Crohn's disease-like ileitis independent of failure in antimicrobial defence.
Schaubeck M, Clavel T, Calasan J, Lagkouvardos I, Haange SB, Jehmlich N, Basic M, Dupont A, Hornef M, von Bergen M, Bleich A, Haller D. Schaubeck M, et al. Gut. 2016 Feb;65(2):225-37. doi: 10.1136/gutjnl-2015-309333. Epub 2015 Apr 17. Gut. 2016. PMID: 25887379 Free PMC article. - RIP3: a molecular switch for necrosis and inflammation.
Moriwaki K, Chan FK. Moriwaki K, et al. Genes Dev. 2013 Aug 1;27(15):1640-9. doi: 10.1101/gad.223321.113. Genes Dev. 2013. PMID: 23913919 Free PMC article. Review.
References
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature reviews Immunol. 2008;8(6):411–420. - PubMed
- Hall PA, et al. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–3577. - PubMed
- Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446(7135):557–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous