The mechanism of action of the histone deacetylase inhibitor vorinostat involves interaction with the insulin-like growth factor signaling pathway - PubMed (original) (raw)
The mechanism of action of the histone deacetylase inhibitor vorinostat involves interaction with the insulin-like growth factor signaling pathway
Rive Sarfstein et al. PLoS One. 2011.
Abstract
A correlation between components of the insulin-like growth factor (IGF) system and endometrial cancer risk has been shown in recent studies. The antitumor action of vorinostat, a histone deacetylase inhibitor, involves changes in the expression of specific genes via acetylation of histones and transcription factors. The aim of this study was to establish whether vorinostat can modify the expression of specific genes related to the IGF-I receptor (IGF-IR) signaling pathway and revert the transformed phenotype. Human endometrioid (Type I, Ishikawa) and uterine serous papillary (Type II, USPC-2) endometrial cancer cell lines were treated with vorinostat in the presence or absence of IGF-I. Vorinostat increased IGF-IR phosphorylation, produced acetylation of histone H3, up-regulated pTEN and p21 expression, and reduced p53 and cyclin D1 levels in Ishikawa cells. Vorinostat up-regulated IGF-IR and p21 expression, produced acetylation of histone H3, and down-regulated the expression of total AKT, pTEN and cyclin D1 in USPC-2 cells. Of interest, IGF-IR activation was associated with a major elevation in IGF-IR promoter activity. In addition, vorinostat treatment induced apoptosis in both cell lines and abolished the anti-apoptotic activity of IGF-I both in the absence or presence of a humanized monoclonal IGF-IR antibody, MK-0646. Finally, vorinostat treatment led to a significant decrease in proliferation and colony forming capability in both cell lines. In summary, our studies demonstrate that vorinostat exhibits a potent apoptotic and anti-proliferative effect in both Type I and II endometrial cancer cells, thus suggesting that endometrial cancer may be therapeutically targeted by vorinostat.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Effect of vorinostat on IGF-I-mediated signal transduction.
Ishikawa and USPC-2 cells were treated for 24 h with vorinostat (or left untreated) and/or IGF-I during the last 10 min of the incubation period. Whole-cell lysates (100 µg) were resolved on SDS-PAGE and immunoblotted with antibodies against pIGF-IR, TIGF-IR, pAKT, TAKT, pERK1/2, TERK1/2, p21, and actin. The figure shows the result of a typical experiment, repeated three times with similar results. IGF-IR phosphorylation in USPC-2 cells was significantly lower than in Ishikawa cells and was detected by Western blot analysis only after longer exposure times of blots to X-ray film. Therefore, this experiment does not allow comparison between both cell lines in terms of levels of expression or phosphorylation.
Figure 2. Effect of vorinostat on apoptosis and acetyl-histone H3.
Western blot analysis of PARP (A) on Ishikawa and USPC-2 cells and caspase 3 (B), Bcl2 (C) and caspase 8 (D) on USPC-2 cells. Cells were treated with vorinostat for 24 h in the absence or presence of IGF-I. After incubation, cell lysates were prepared and electrophoresed (100 µg for Bcl2 or 70 µg for caspases 3 and 8). Caspase 3 expression in USPC-2 cells was detected after a long exposure time of blots to X-ray film. Results are representative of three independent experiments.
Figure 3. Effect of vorinostat on p53 and pTEN expression.
Ishikawa and USPC-2 cells were treated for 24 h with vorinostat (or left untreated) and/or IGF-I. Whole-cell lysates (100 µg) were resolved on SDS-PAGE and immunoblotted with antibodies against p53, pTEN, acetyl-histone H3 and actin. Western blot analysis revealed that basal p53 levels were much lower in USPC-2 than in Ishikawa cells and were detected after long exposure times. Results represent a typical experiment out of three independent experiments.
Figure 4. Regulation of IGF-IR promoter activity by vorinostat in endometrial cancer cells.
Ishikawa and USPC-2 cells were transiently transfected with an IGF-IR promoter-luciferase reporter plasmid, p(−476/+640)LUC, which contains most of the proximal region of the IGF-IR promoter, and a ß-galactosidase vector. Promoter activity is expressed as luciferase values normalized for ß-galactosidase. A value of 100% was given to the promoter activity in the absence of vorinostat. Results are mean ± S.E.M. (three independent experiments); p*<0.01 versus untreated cells.
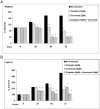
Figure 5. Effect of vorinostat on endometrial cancer cells proliferation.
Cells were plated in 24-well plates at a density of 2×104 cells/well for Ishikawa (A) and 3×104 cells/well for USPC-2 (B). The bars represent the mean ± S.E.M. of three independent experiments, performed each in triplicate; * p<0.05 versus untreated cells.
Figure 6. Effect of vorinostat on the mTor and Ampk signaling pathway in endometrial cancer cells.
Ishikawa and USPC-2 cells were treated with vorinostat and/or IGF-I, and cell lysates (100 µg) were prepared after 24 h. Western blot analysis was performed with antibodies against pmTor, T-mTor, p-Ampk, T-Ampk, p21, cyclin D1, PI3K and actin. The figure shows the results of a typical experiment, repeated three times with similar results.
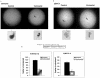
Figure 7. Colony formation in soft agar.
A total of 1.5×104 Ishikawa cells and 1.2×104 USPC-1 cells were seeded on 6-well plates in soft agar. The formed colonies were photographed at a magnification of 40× and 200× (A) and colonies with diameters greater than 3 mm were counted (B) at day 22. The bars represent the mean ± S.E.M. of three independent wells; * p<0.05 versus untreated cells.
Figure 8. Effect of vorinostat on wound healing assays in endometrial cancer cells.
Wounds were made on monolayers of Ishikawa (A) and USPC-2 (B) cells grown to 100% confluence. Untreated (control) cells or cells treated with vorinostat and/or IGF-I were photographed just after scratch (time 0), and after 48 h and 72 h. Results presented here are representative of triplicate independent samples of each cell line.
Figure 9. Effect of vorinostat and/or antibody MK-0646 on apoptosis and IGF-IR protein levels.
Ishikawa and USPC-2 cells were treated with vorinostat for 24 h, separately and in combination with MK-0646, in the presence or absence of IGF-I. Whole-cell lysates (70 µg for caspase 9 and 100 µg for total IGF-IR and p21 proteins detection) were resolved by SDS-PAGE and immunoblotted with antibodies against T-IGF-IR and p21 (A) and caspase 9 (B). The figure shows the results of a typical experiment, repeated twice with similar results.
Similar articles
- Genetic blockade of insulin-like growth factor-1 receptor via recombinant adenovirus in lung cancer can be enhanced by the histone deacetylase inhibitor, vorinostat.
Park MY, Kim DR, Eo EY, Lim HJ, Park JS, Cho YJ, Yoon HI, Lee JH, Lee CT. Park MY, et al. J Gene Med. 2013 Mar-Apr;15(3-4):115-22. doi: 10.1002/jgm.2699. J Gene Med. 2013. PMID: 23413016 - Activation of insulin-like growth factor receptor signaling mediates resistance to histone deacetylase inhibitors.
Kim JS, Lee SC, Min HY, Park KH, Hyun SY, Kwon SJ, Choi SP, Kim WY, Lee HJ, Lee HY. Kim JS, et al. Cancer Lett. 2015 Jun 1;361(2):197-206. doi: 10.1016/j.canlet.2015.02.038. Epub 2015 Feb 23. Cancer Lett. 2015. PMID: 25721083 - DNA microarray profiling of genes differentially regulated by the histone deacetylase inhibitors vorinostat and LBH589 in colon cancer cell lines.
LaBonte MJ, Wilson PM, Fazzone W, Groshen S, Lenz HJ, Ladner RD. LaBonte MJ, et al. BMC Med Genomics. 2009 Nov 30;2:67. doi: 10.1186/1755-8794-2-67. BMC Med Genomics. 2009. PMID: 19948057 Free PMC article. - Human endometrial cytodifferentiation by histone deacetylase inhibitors.
Uchida H, Maruyama T, Nagashima T, Ono M, Masuda H, Arase T, Sugiura I, Onouchi M, Kajitani T, Asada H, Yoshimura Y. Uchida H, et al. Hum Cell. 2006 Feb;19(1):38-42. doi: 10.1111/j.1749-0774.2005.00006.x. Hum Cell. 2006. PMID: 16643606 Review. - Targeting histone deacetylases: development of vorinostat for the treatment of cancer.
Richon VM. Richon VM. Epigenomics. 2010 Jun;2(3):457-65. doi: 10.2217/epi.10.20. Epigenomics. 2010. PMID: 22121904 Review.
Cited by
- Targeting Epigenetic Regulators for Endometrial Cancer Therapy: Its Molecular Biology and Potential Clinical Applications.
Inoue F, Sone K, Toyohara Y, Takahashi Y, Kukita A, Hara A, Taguchi A, Tanikawa M, Tsuruga T, Osuga Y. Inoue F, et al. Int J Mol Sci. 2021 Feb 25;22(5):2305. doi: 10.3390/ijms22052305. Int J Mol Sci. 2021. PMID: 33669072 Free PMC article. Review. - Vorinostat in combination with bortezomib in patients with advanced malignancies directly alters transcription of target genes.
Kolesar JM, Traynor AM, Holen KD, Hoang T, Seo S, Kim K, Alberti D, Espinoza-Delgado I, Wright JJ, Wilding G, Bailey HH, Schelman WR. Kolesar JM, et al. Cancer Chemother Pharmacol. 2013 Sep;72(3):661-7. doi: 10.1007/s00280-013-2242-6. Epub 2013 Aug 1. Cancer Chemother Pharmacol. 2013. PMID: 23903894 Free PMC article. Clinical Trial. - Histone deacetylases, microRNA and leptin crosstalk in pancreatic cancer.
Tchio Mantho CI, Harbuzariu A, Gonzalez-Perez RR. Tchio Mantho CI, et al. World J Clin Oncol. 2017 Jun 10;8(3):178-189. doi: 10.5306/wjco.v8.i3.178. World J Clin Oncol. 2017. PMID: 28638788 Free PMC article. Review. - Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior.
Lloyd BA, Hake HS, Ishiwata T, Farmer CE, Loetz EC, Fleshner M, Bland ST, Greenwood BN. Lloyd BA, et al. Behav Brain Res. 2017 Apr 14;323:56-67. doi: 10.1016/j.bbr.2017.01.033. Epub 2017 Jan 24. Behav Brain Res. 2017. PMID: 28130174 Free PMC article.
References
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. - PubMed
- Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004;444:213–223. - PubMed
- Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994;54:264–268. - PubMed
- Pallares J, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, et al. Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J Pathol. 2004;204:569–577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous