Ubiquitination in the first cytoplasmic loop of μ-opioid receptors reveals a hierarchical mechanism of lysosomal down-regulation - PubMed (original) (raw)
Ubiquitination in the first cytoplasmic loop of μ-opioid receptors reveals a hierarchical mechanism of lysosomal down-regulation
James N Hislop et al. J Biol Chem. 2011.
Abstract
μ-Type opioid receptors (MORs) are members of the large seven-transmembrane receptor family which transduce the effects of both endogenous neuropeptides and clinically important opioid drugs. Prolonged activation of MORs promotes their proteolytic degradation by endocytic trafficking to lysosomes. This down-regulation process is known to contribute to homeostatic regulation of cellular opioid responsiveness, but mechanisms that mediate and control MOR down-regulation have not been defined. We show here that lysosomal down-regulation of MORs is ESCRT (endosomal sorting complex required for transport)-dependent and involves ubiquitin-promoted transfer of internalized MORs from the limiting endosome membrane to lumen. We also show that MOR down-regulation measured by conventional radioligand binding assay is determined specifically by ubiquitination in the first cytoplasmic loop. Surprisingly, we were unable to find any role of ubiquitination in determining whether internalized receptors recycle or are delivered to lysosomes. Instead, this decision is dictated specifically by the MOR C-tail and occurs irrespectively of the presence or absence of receptor ubiquitination. Our results support a hierarchical organization of discrete ubiquitin-independent and -dependent sorting operations, which function non-redundantly in the conserved down-regulation pathway to mediate precise endocytic control. Furthermore, they show that this hierarchical mechanism discriminates the endocytic regulation of naturally occurring MOR isoforms. Moreover, they are the first to reveal, we believe, for any seven-transmembrane receptor, a functional role of ubiquitination in the first cytoplasmic loop.
Figures
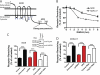
FIGURE 1.
Both MOR and MORΔ17 undergo ESCRT-dependent down-regulation by lysosomes. A, shown is a diagrammatic representation of the F-MOR and F-MORΔ17 constructs, indicating the location of cytoplasmic lysine residues and the previously identified MRS. B, shown is the time course of the down-regulation of F-MOR and F-MORΔ17. HEK293 cells stably expressing either receptor were incubated at 37 °C with 10 μ
m
DADLE for the indicated time period, and then radioligand binding assay was carried out using the high affinity radiolabeled opioid antagonist [3H]DPN at 10 n
m
to estimate Bmax, as described under “Experimental Procedures.” Data points represent specific binding of [3H]DPN measured at each time point, expressed as a percentage of the specific binding in cells not exposed to agonist. Points represent mean determinations from independent experiments, with each time point analyzed in triplicate tubes in each experiment. Error bars represent the S.E. calculated across the experiments (n = 3–5). C and D, shown is the effect of the indicated experimental manipulations on DADLE-induced down-regulation of F-MOR (panel C) and F-MORΔ17 (panel D) in stably transfected HEK293 cells. Cells were transfected with “empty” pcDNA3 or pcDNA3-HRS (red bars) or transfected with control (scrambled) siRNA or siRNA targeting TSG101 (white bars). A final set of cells was pretreated with 200 μ
m
chloroquine for 20 min before the start of the experiment (black bars). Cells were then left untreated or exposed to 10 μ
m
DADLE for 5 h (F-MORΔ17, panel D) or 8 h (F-MOR, panel C), chosen according to the different down-regulation kinetics of these constructs, before carrying out the radioligand binding assay using 10 n
m
[3H]DPN. Bars represent the specific binding detected after agonist treatment, expressed as a percentage of that detected to identically manipulated cells except not exposed to agonist. In each experiment binding was determined in triplicate tubes. Bars represent average determinations, and error bars S.E., across multiple experiments (n = 3–6; ***, p = <0.001, two way ANOVA). The inset shows correlative immunoblot data verifying Tsg101 depletion by the siRNA.
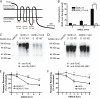
FIGURE 2.
MOR down-regulation measured radioligand binding requires receptor ubiquitination. A, shown is a diagram of the F-MOR-0cK construct indicating cytoplasmic domains containing the Lys → Arg mutation (R) as well as the MRS that is devoid of lysine residues. icl, cytoplasmic loop. B, shown is a densitometric analysis of ubiquitin incorporation into the F-MORΔ17 and the lysyl-mutant F-MORΔ17–0cK, assessed after immunopurification of receptors in the presence of SDS. Data shown represent the mean and S.E. of densitometry from anti-ubiquitin immunoblot analysis carried out in n = 3 independent experiments. Scanning densitometry was carried out in the linear range and is expressed as -fold over background (defined by nonspecific signal detected in parental HEK293 cells not expressing the indicated FLAG-tagged receptor). C, a representative anti-ubiquitin immunoblot (IB) from the analysis summarized in panel B; IP, immunoprecipitate. D, shown is the same blot as in panel C, except it was stripped and reprobed with anti-FLAG to verify comparable loading and transfer of immunopurified receptors. E and F, shown is the effect of the indicated lysyl mutations on DADLE-induced down-regulation. HEK293 cells stably expressing F-MORΔ17 or F-MORΔ17–0cK (panel E) or F-MOR or F-MOR-0cK (panel F) were exposed to 10 μ
m
DADLE for the indicated time period before estimating Bmax by radioligand binding to [3H]DPN. Points represent specific binding at each time point, expressed as a percentage of the specific binding in cells not exposed to agonist. In each experiment triplicate tubes were analyzed; points represent averages and error bars S.E. across experiments (n = 4; *, p < 0.05; ***, p < 0.001, two way ANOVA).
FIGURE 3.
MOR trafficking to lysosomes, assessed biochemically or immunochemically, does not require receptor ubiquitination and is dictated by the C-tail. A, representative anti-FLAG immunoblots (from three-six independent experiments) show the effects of exposing cells to 10 μ
m
DADLE for the indicated time period on FLAG-tagged receptor signal detected in cell extracts. Stably transfected HEK293 cells initially expressing similar levels of F-MOR (left), F-MORΔ17 (middle), or F-MORΔ17–0cK (right) were analyzed. Numbers above each lane indicate the time period of DADLE incubation in hours. B, shown is a comparison of recycling between F-MOR (black) F-MORΔ17 (blue) and F-MORΔ17–0cK (red). Stably transfected cells expressing the indicated receptor construct were incubated for 30 min in the presence of 10 μ
m
DADLE to drive endocytosis and then washed and incubated at 37 °C in the presence of 10 μ
m
naloxone for the indicated times before surface labeling of Alexa647-conjugated M1 anti-FLAG and quantifying surface receptor immunoreactivity by flow cytometry. Displayed are the proportion of internalized receptors that recovered to the plasma membrane at the indicated time point after agonist washout, calculated as described under “Experimental Procedures” (mean ± S.E., n = 3–4; *, p < 0.05, two-way ANOVA, Bonferroni post-test). C, shown are representative confocal micrographs showing the localization of F-MORΔ17 (i–iii) or F-MORΔ17–0cK (iv–vi) relative to LAMP1/2 immunoreactivity in stably transfected HEK293 cells fixed after exposure to 10 μ
m
DADLE for 90 min. Merged images (iii and vii) display receptor and LAMP channels pseudocolored in green and magenta, respectively, with areas of colocalization appearing white. Insets show a magnified region of the image as illustrated by the dotted box. Arrows indicate puncta that appear to be only single colors due to differences in relative intensity of one or the other label but are in colocalized when examined at the level of individual images. D, quantification of agonist-induced proteolysis of F-MORΔ17 and F-MORΔ17–0cK, derived from multiple experiments corresponding to the example shown in panel A, were determined by exposure of anti-FLAG immunoblots in the linear detection range and scanning densitometry. Points indicate mean and error bars S.E. (n = 6 experiments).
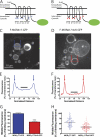
FIGURE 4.
Ubiquitination promotes receptor redistribution from the limiting to intralumenal endosome membranes. A and B, shown are schematic representations of the F-MORΔ17-GFP (panel A) and F-MORΔ17–0cK-GFP (panel B) constructs used for live imaging, with the fused GFP indicated as a green oval. icl, cytoplasmic loop. C and D, a representative optical section shows endosomes in HEK293 cells co-transfected with CFP-Rab5Q79L and either F-MORΔ17-GFP (panel C) or F-MORΔ17–0cK-GFP (panel D) after incubation for 90 min at 37 °C with 10 μ
m
DADLE followed by imaging at 37 °C by spinning disc confocal microscopy in the continuous presence of agonist. Essentially all of the enlarged endosomes contained visible F-MOR-GFP fluorescence, whereas intralumenal F-MORΔ17–0cK-GFP was rarely observed. The asterisk in panel D shows an example of such a rare endosome in which detectable intralumenal F-MORΔ17–0cK-GFP fluorescence was detected. Blue and red symbols overlaid on each image indicate the position of line scans used for quantification. Scale bars represent 10 μm. E and F, shown is a representative line scan analysis to quantify intralumenal fluorescence. Normalized distance represents the diameter of the endosome shown, where 0 and 100 correspond to the pixel distances between the first and second maximum pixel intensities measured across the dashed line, respectively (indicated by arrows in the diagrams overlaid on panels C and D). Normalized fluorescence represents the normalized pixel intensity measured across the dashed line, where the maximum pixel intensity across the line is normalized to 100. The graphs shown in panels E and F show line scans of the representative endosomes highlighted in panels A and B, respectively. The black line highlights the normalized fluorescence values of pixels from 40 to 60% of the normalized diameter, used for determining the mean value of intralumenal fluorescence for each endosome, as described under “Experimental Procedures,” G and H, shown are compiled results from the line scan analysis diagrammed in panels E and F. Panel G shows compiled data as the mean and S.E. Panel H shows the distribution of internal fluorescence values from individual analyzed endosomes (n = 98 and 109 endosomes, respectively, each imaged from 12 independent dishes and cells; ***, p < 0.001, Student's t test).
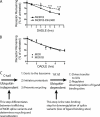
FIGURE 5.
Ubiquitination specifically of the first cytoplasmic loop is necessary and sufficient for agonist-induced down-regulation measured by radioligand binding. A, shown is a diagram of the series of receptor mutants used to test sufficiency, based on reverting arginine residues to lysine residues within individual cytoplasmic domains of the F-MORΔ17–0cK backbone separately. The positions of lysine (K) or arginine (R) residues in each construct are indicated. B, shown is a diagram of the receptor mutant used to test necessity, based on mutating only the lysine residues present in the first cytoplasmic loop (1st icl) of F-MORΔ17 to arginine, as indicated. C, shown is down-regulation of the receptor constructs diagrammed in panel A, assayed by [3H]DPN binding after incubation of stably transfected cells expressing the indicated receptor construct with 10 μ
m
DADLE for 5 h. In each experiment down-regulation was assessed in triplicate determinations. Bars represent the mean, and error bars are from n = 7 independent experiments (**, p < 0.01; ***, p < 0.001, one way ANOVA, Bonferroni post test). D, shown is down-regulation of the receptor construct diagrammed in panel B. E, shown is a representative anti-ubiquitin immunoblot (IB) used for the densitometry analysis summarized in panels F and G. IP, immunoprecipitate. F and G, shown is quantification of relative ubiquitin incorporation from densitometry of anti-ubiquitin blots expressed as -fold over basal measured in unstimulated cells (panel G) or -fold over background measured in cells not expressing FLAG-tagged receptor (panel F). Bars represent mean and S.E.; error bars, n = 4; * denotes p < 0.05 as determined by two-way ANOVA with the Bonferroni post-test). H, the same blot is shown in panel E except it was stripped and reprobed with anti-FLAG, to verify comparable receptor loading between lanes.
FIGURE 6.
Ubiquitination in the first cytoplasmic loop is also required for pharmacological down-regulation of the MOR1B isoform. A, HEK293 cells stably expressing FLAG-tagged MOR1B (F-MOR1B) or FLAG-tagged MOR1B in which only the lysine residues present in the first cytoplasmic loop were mutated to arginine (F-MOR1B-K94R,K96R) and treated with 10 μ
m
DADLE for the indicated time period before assessing receptor down-regulation by radioligand binding using [3H]DPN. Points represent mean determinations from independent experiments, with each time point analyzed in triplicate tubes in each experiment. Error bars represent the S.E. calculated across the experiments (n = 4). B, shown is a down-regulation assay comparing the MOR1 and the MOR1B splice variants. Data are replotted from Figs. 1_B_ and 6_A_ to reveal that, in the present experiments, there was no detectable difference in pharmacological down-regulation of the wild type versions of MOR1 (F-MOR) compared with MOR1B (F-MOR1B) isoforms. C, shown is a diagram describing the proposed sequential sorting operations in the hierarchical sorting model. The C-tail (containing the previously described MRS) determines the overall trafficking itinerary of internalized MORs between recycling and lysosomal routes and does not require MOR ubiquitination. Ubiquitination of the first cytoplasmic loop (1st icl) specifically promotes redistribution of receptors from the limiting membrane to lumen of late endosomes/multivesicular bodies. This intra-multivesicular body “topological” sorting operation does not dictate the overall trafficking itinerary of internalized receptors but is required for efficient destruction of the transmembrane helical bundle containing the diprenorphine binding site. This step is effectively rate-limiting for pharmacological down-regulation of both the MOR1 and MOR1B isoforms. Therefore, traditional down-regulation assays based on loss of radioligand binding sites may not be sensitive to functionally significant differences in the regulated endocytic trafficking itinerary of naturally occurring MOR isoforms.
Similar articles
- The role of ubiquitination in lysosomal trafficking of δ-opioid receptors.
Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN. Henry AG, et al. Traffic. 2011 Feb;12(2):170-84. doi: 10.1111/j.1600-0854.2010.01145.x. Epub 2010 Dec 22. Traffic. 2011. PMID: 21106040 Free PMC article. - Facilitation of μ-opioid receptor activity by preventing δ-opioid receptor-mediated codegradation.
He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H, Luo J, Li ZY, Wang Q, Lu YJ, Bao L, Zhang X. He SQ, et al. Neuron. 2011 Jan 13;69(1):120-31. doi: 10.1016/j.neuron.2010.12.001. Neuron. 2011. PMID: 21220103 - Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes.
Marley A, von Zastrow M. Marley A, et al. PLoS One. 2010 Feb 19;5(2):e9325. doi: 10.1371/journal.pone.0009325. PLoS One. 2010. PMID: 20174469 Free PMC article. - Role of ubiquitination in endocytic trafficking of G-protein-coupled receptors.
Hislop JN, von Zastrow M. Hislop JN, et al. Traffic. 2011 Feb;12(2):137-48. doi: 10.1111/j.1600-0854.2010.01121.x. Epub 2010 Oct 15. Traffic. 2011. PMID: 20854416 Free PMC article. Review. - Ubiquitin and endocytic protein sorting.
Urbé S. Urbé S. Essays Biochem. 2005;41:81-98. doi: 10.1042/EB0410081. Essays Biochem. 2005. PMID: 16250899 Review.
Cited by
- Polarized human cholangiocytes release distinct populations of apical and basolateral small extracellular vesicles.
Davies BA, Morton LO, Jefferson JR, Rozeveld CN, Doskey LC, LaRusso NF, Katzmann DJ. Davies BA, et al. Mol Biol Cell. 2020 Oct 15;31(22):2463-2474. doi: 10.1091/mbc.E19-03-0133. Epub 2020 Aug 26. Mol Biol Cell. 2020. PMID: 32845745 Free PMC article. - Regulation of endocytic clathrin dynamics by cargo ubiquitination.
Henry AG, Hislop JN, Grove J, Thorn K, Marsh M, von Zastrow M. Henry AG, et al. Dev Cell. 2012 Sep 11;23(3):519-32. doi: 10.1016/j.devcel.2012.08.003. Epub 2012 Aug 30. Dev Cell. 2012. PMID: 22940114 Free PMC article. - Regulation of G Protein-Coupled Receptors by Ubiquitination.
Skieterska K, Rondou P, Van Craenenbroeck K. Skieterska K, et al. Int J Mol Sci. 2017 Apr 27;18(5):923. doi: 10.3390/ijms18050923. Int J Mol Sci. 2017. PMID: 28448471 Free PMC article. Review. - Ubiquitin chains earmark GPCRs for BBSome-mediated removal from cilia.
Shinde SR, Nager AR, Nachury MV. Shinde SR, et al. J Cell Biol. 2020 Dec 7;219(12):e202003020. doi: 10.1083/jcb.202003020. J Cell Biol. 2020. PMID: 33185668 Free PMC article. - Response of striosomal opioid signaling to dopamine depletion in 6-hydroxydopamine-lesioned rat model of Parkinson's disease: a potential compensatory role.
Koizumi H, Morigaki R, Okita S, Nagahiro S, Kaji R, Nakagawa M, Goto S. Koizumi H, et al. Front Cell Neurosci. 2013 May 17;7:74. doi: 10.3389/fncel.2013.00074. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23730270 Free PMC article.
References
- Koch T., Höllt V. (2008) Pharmacol. Ther. 117, 199–206 - PubMed
- Martini L., Whistler J. L. (2007) Curr. Opin Neurobiol. 17, 556–564 - PubMed
- Williams J. T., Christie M. J., Manzoni O. (2001) Physiol. Rev. 81, 299–343 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DA010711/DA/NIDA NIH HHS/United States
- DA012864/DA/NIDA NIH HHS/United States
- R29 DA010711/DA/NIDA NIH HHS/United States
- DA010711/DA/NIDA NIH HHS/United States
- R01 DA012864/DA/NIDA NIH HHS/United States
- R01 DA010711/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials