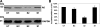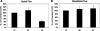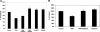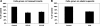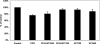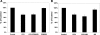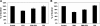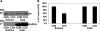Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells - PubMed (original) (raw)
Comparative Study
Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells
Hatim A Hassan et al. Am J Physiol Cell Physiol. 2012.
Abstract
Urolithiasis remains a very common disease in Western countries. Seventy to eighty percent of kidney stones are composed of calcium oxalate, and minor changes in urinary oxalate affect stone risk. Intestinal oxalate secretion mediated by anion exchanger SLC26A6 plays a major constitutive role in limiting net absorption of ingested oxalate, thereby preventing hyperoxaluria and calcium oxalate urolithiasis. Using the relatively selective PKC-δ inhibitor rottlerin, we had previously found that PKC-δ activation inhibits Slc26a6 activity in mouse duodenal tissue. To identify a model system to study physiologic agonists upstream of PKC-δ, we characterized the human intestinal cell line T84. Knockdown studies demonstrated that endogenous SLC26A6 mediates most of the oxalate transport by T84 cells. Cholinergic stimulation with carbachol modulates intestinal ion transport through signaling pathways including PKC activation. We therefore examined whether carbachol affects oxalate transport in T84 cells. We found that carbachol significantly inhibited oxalate transport by T84 cells, an effect blocked by rottlerin. Carbachol also led to significant translocation of PKC-δ from the cytosol to the membrane of T84 cells. Using pharmacological inhibitors, we observed that carbachol inhibits oxalate transport through the M(3) muscarinic receptor and phospholipase C. Utilizing the Src inhibitor PP2 and phosphorylation studies, we found that the observed regulation downstream of PKC-δ is partially mediated by c-Src. Biotinylation studies revealed that carbachol inhibits oxalate transport by reducing SLC26A6 surface expression. We conclude that carbachol negatively regulates oxalate transport by reducing SLC26A6 surface expression in T84 cells through signaling pathways including the M(3) muscarinic receptor, phospholipase C, PKC-δ, and c-Src.
Figures
Fig. 1.
Functional characterization of T84 cells. T84 cells were first incubated as described in
materials and methods
in NaCl solution for 30 min, which then was replaced with a Cl-free solution [intracellular Cl (Cli) > extracellular Cl (Clo)] in the absence of (gluconate) or presence of 100 μM of the anion exchange inhibitor DIDS (gluconate + DIDS), or a chloride solution (Clo > Cli) containing [14C]oxalate for 6 min. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective control (gluconate) value ([14C]oxalate uptake rate = 3.04 ± 0.75 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. Both DIDS and the presence of chloride (Clo > Cli) significantly inhibited [14C]oxalate uptake (P < 0.001, by ANOVA).
Fig. 2.
SLC26A6 (A6) knockdown in T84 cells using short hairpin RNA (shRNA). A: representative Western blot analysis of SLC26A6 protein expression. SLC26A6 protein expression was evaluated in T84 cell lysate (30 μg protein/lane: UT, untransfected cells; NC, T84 cells transfected with the negative control shRNA; S1 and S2, T84 cells transfected with shRNAs targeting SLC26A6). The lower half of the same blot was probed with an anti-GAPDH antibody to normalize loading of protein in each lane (bottom). B: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software [National Institutes of Health (NIH, Bethesda, MD)]. Values are means ± SE of 7 different experiments of relative SLC26A6 abundance to GAPDH and are presented as a percentage of the respective control (UT) value. shRNA knockdown of SLC26A6 (S2) significantly reduced SLC26A6 total protein expression (P < 0.001 compared with UT and NC, by ANOVA).
Fig. 3.
Effect of SLC26A6 knockdown on [14C]oxalate uptake by T84 cells. Apical (A) and basolateral (B) [14C]oxalate uptake by UT, NC, and S2 was measured as described in
materials and methods
. Values are means ± SE of 5 independent experiments each of which was normalized to the respective control (UT) value ([14C]oxalate uptake rate = 2.14 ± 0.57 and 1.53 ± 0.47 pmol·cm−2·min−1, apical and basolateral, respectively). All experiments were performed on transwell-grown cells. SLC26A6 knockdown significantly reduced apical [14C]oxalate uptake (P < 0.05 compared with UT and NC, by ANOVA), but had no effect on basolateral [14C]oxalate uptake.
Fig. 4.
Effect of PKC activation on [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control) or 200 nM PMA (PMA) for 45 min (× 15 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in
materials and methods
. [14C]Oxalate uptake was also measured in the presence of Gö6976 (100 nM) or Gö6983 (5 μM) for 60 min followed by 200 nM PMA with continued presence of Gö6976 (PMA + Gö6976) or Gö6983 (PMA + Gö6983), or 100 nM Gö6976 (Gö6976) or 5 μM Gö6983 (Gö6983) alone for 105 min. Values are means ± SE of 5 independent experiments (3 on plastic- and 2 on transwell-grown cells) each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 2.09 ± 0.60 pmol·cm−2·min−1). Gö6983 significantly reduced the inhibition induced by PMA (P < 0.01, by ANOVA). B: effect of rottlerin on PMA-induced inhibition of [14C]oxalate uptake by T84 cells. T84 cells were preincubated with vehicle (control) or 200 nM PMA (PMA) for 45 min (× 15 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in
materials and methods
. [14C]Oxalate uptake was also measured in the presence of rottlerin (10 μM) for 60 min followed by 200 nM PMA with continued presence of rottlerin (PMA + rottlerin), or 10 μM rottlerin alone for 105 min (rottlerin). Values are means ± SE of 5 independent experiments (3 on plastic- and 2 on transwell-grown cells) each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.64 ± 0.29 pmol·cm−2·min−1). Rottlerin significantly reduced the inhibition induced by PMA (P < 0.01, by ANOVA).
Fig. 5.
Effect of carbachol (CCH) on [14C]oxalate uptake by T84 cells. A: T84 cells grown on transwell inserts were preincubated with vehicle (control) or 150 μM carbachol (apically = CCH-A or basolaterally = CCH-B) for 90 min (× 60 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 4 independent experiments each of which was normalized to the respective control value ([14C]oxalate uptake rate = 2.42 ± 0.61 pmol·cm−2·min−1). Carbachol significantly reduced [14C]oxalate uptake (P < 0.001 apically and basolaterally, by ANOVA). B: T84 cells grown on plastic supports were preincubated with vehicle (control) or 150 μM carbachol for 90 min (× 60 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 4 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.04 ± 0.15 pmol·cm−2·min−1). Carbachol significantly reduced [14C]oxalate uptake (P < 0.0007, two-tailed _t_-test).
Fig. 6.
Effect of CFTR inhibitor-172 (CFTR-172) on carbachol-induced inhibition of [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 10 μM CFTR inhibitor-172 for 15 min followed by 150 μM carbachol for 90 min (CCH + CFTR-172), or 10 μM CFTR inhibitor-172 alone for 15 min (CFTR-172), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 5 independent experiments each of which was done in duplicate or triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 0.94 ± 0.12 pmol·cm−2·min−1). All experiments were performed on transwell-grown cells. CFTR inhibitor-172 has no significant effect on the inhibition induced by carbachol. B: effect of CFTR inhibitor-172 on carbachol-induced chloride secretion [measured as changes in short-circuit current (Δ_I_sc)] across T84 cells. T84 cell monolayers (seeded at the same time and studied concurrently as in A) were mounted in Ussing chambers as described in
materials and methods
. Following a 15-min equilibration period, vehicle (control) or 10 μM CFTR inhibitor-172 was added to the mucosal side of matched pairs of monolayers for 15 min, followed by the addition of carbachol (150 μM) to the serosal side to elicit chloride secretion. Shown here is the peak _I_sc elicited by carbachol. Values are means ± SE of 7 monolayers per group. CFTR inhibitor-172 significantly inhibited carbachol-induced chloride secretion (Δ_I_sc) (P < 0.001, by ANOVA).
Fig. 7.
Effect of rottlerin on carbachol-induced inhibition of [14C]oxalate uptake by T84 cells. T84 cells were preincubated with vehicle (control) or 150 μM carbachol for 90 min (× 60 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in
materials and methods
. [14C]Oxalate uptake was also measured in the presence of 10 μM rottlerin for 60 min followed by 150 μM carbachol with continued presence of rottlerin for 90 min (CCH + rottlerin), or 10 μM rottlerin alone for 150 min (rottlerin). Values are means ± SE of 5 independent experiments (4 on plastic- and 1 on transwell-grown cells) each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.17 ± 0.43 pmol·cm−2·min−1). Rottlerin significantly reduced the inhibition induced by carbachol (P < 0.05, by ANOVA).
Fig. 8.
Effect of carbachol on PKC-δ translocation in T84 cells. A: representative Western blot analysis of PKC-δ (60 μg protein/lane). T84 cells were preincubated with vehicle (control) or 150 μM carbachol for 90 min before cytosolic (C) and membrane (M) fractions were collected as described in
materials and methods
. B: densitometry of immunoblot results. Western blot band density was quantified using NIH Scion Image software. Values are means ± SE of 4 different experiments each of which was normalized to total blotted PKC-δ (C + M) under each condition (control or CCH). All experiments were performed on plastic-grown cells. Carbachol led to significant translocation of PKC-δ from the cytosolic to the membrane fraction of T84 cells (P < 0.001 compared with the cytosolic and membrane fractions in the control, by ANOVA).
Fig. 9.
Effect of muscarinic receptor blockade on carbachol-induced inhibition of [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 1 μM atropine (ATR: a nonspecific muscarinic receptor antagonist) for 15 min followed by 150 μM carbachol with continued presence of atropine for 90 min (CCH + ATR), or 1 μM atropine alone for 105 min (ATR), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.73 ± 0.30 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. Atropine significantly reduced the inhibition induced by carbachol (P < 0.01, by ANOVA). B: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 200 nM 4-diphenylacetoxy-_N_-methylpiperidine methiodide (4-DAMP: an M3 muscarinic receptor antagonist) for 15 min followed by 150 μM carbachol with continued presence of 4-DAMP for 90 min (CCH + DAMP), or 200 nM 4-DAMP alone for 105 min (DAMP), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 6 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.17 ± 0.22 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. 4-DAMP significantly reduced the inhibition induced by carbachol (P < 0.001, by ANOVA).
Fig. 10.
Effect of the phospholipase C inhibitor U73122 and its inactive analog U73343 on carbachol-induced inhibition of [14C]oxalate uptake by T84 cells. T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 10 μM U73122 or U73343 for 30 min followed by 150 μM carbachol with continued presence of U73122 (CCH + U73122) or U73343 (CCH + U73343) for 90 min, or 10 μM U73122 (U73122) or U73343 (U73343) alone for 120 min, and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 9 independent experiments (6 on plastic- and 3 on transwell-grown cells) each of which was done in duplicate or triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 2.08 ± 0.34 pmol·cm−2·min−1). U73122 significantly reduced the inhibition induced by carbachol (P < 0.05, by ANOVA).
Fig. 11.
Effect of ERK1/2 and p38 inhibition on carbachol-induced suppression of [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, or 10 μM of the MEK/ERK1/2 inhibitor PD98059 for 15 min followed by 150 μM carbachol with continued presence of PD98059 for 90 min (CCH + PD98059), or 10 μM PD98059 alone for 105 min (PD98059), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.12 ± 0.26 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. B: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, or 10 μM of the p38 inhibitor SB 202190 for 60 min followed by 150 μM carbachol with continued presence of SB 202190 for 90 min (CCH + SB), or 10 μM SB 202190 alone for 150 min (SB), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.57 ± 0.59 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. PD98059 and SB 202190 had no significant effect on the inhibition induced by carbachol.
Fig. 12.
Effect of the Src family kinase inhibitor PP2 on carbachol- and PMA-induced inhibition of [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 10 μM PP2 for 60 min followed by 150 μM carbachol with continued presence of PP2 (CCH + PP2), or 10 μM PP2 alone for 150 min (PP2), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 8 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 2.88 ± 0.56 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. PP2 significantly reduced the inhibition induced by carbachol (P < 0.05, by ANOVA). B: T84 cells were preincubated with vehicle (control), or 200 nM PMA (PMA) for 45 min, 10 μM PP2 for 60 min followed by 200 nM PMA with continued presence of PP2 (PMA + PP2), or 10 μM PP2 alone for 105 min (PP2), and then [14C]oxalate uptake was measured as described in
materials and methods
. Values are means ± SE of 5 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 0.90 ± 0.17 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. PP2 significantly reduced the inhibition induced by PMA (P < 0.01, by ANOVA).
Fig. 13.
Effect of carbachol on c-Src phosphorylation in T84 cells. A: representative Western blot analysis of phospho-c-Src (p c-Src) in T84 cells. T84 cells were preincubated with vehicle (control) or 150 μM carbachol for 90 min before total protein lysates were collected as described in
materials and methods
. Equal amounts of protein lysates from vehicle (control)- and carbachol-treated T84 cells were immunoprecipitated with an anti-c-Src, and then immunoblots were prepared and probed with phospho-Src family (Tyr416) antibody. The blot was stripped and reprobed with an anti-c-Src (c-Src) antibody to verify equal loading of protein in each lane (bottom). B: densitometry of immunoblot results. Western blot band density was quantified using NIH Scion Image software. Values are means ± SE of 5 different experiments and are expressed as relative arbitrary units. All experiments were performed on plastic-grown cells. Carbachol led to significant stimulation of c-Src phosphorylation in T84 cells (P < 0.02, two-tailed _t_-test).
Fig. 14.
Effect of carbachol on SLC26A6 (A6) protein expression assayed by immunoblotting. A: representative Western blot analysis of total and surface biotinylated SLC26A6. T84 cells were preincubated with vehicle (control, CON) or 150 μM carbachol for 90 min, and then SLC26A6 protein expression was evaluated in cell lysate (total: 20 μg protein/lane) and after streptavidin precipitation of surface biotinylated proteins from 1,500 μg of initial cell lysate (Surface) performed as described in
materials and methods
. The lower half of the same blot was probed with an anti-GAPDH antibody to verify equal loading of protein in each lane (bottom). B: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means ± SE for five different experiments (2 on plastic- and 3 on transwell-grown cells) each of which was normalized to the respective control value. Carbachol significantly reduced the amount of SLC26A6 protein available to surface biotinylation (P < 0.001, by ANOVA).
Comment in
- This, too, shall pass--like a kidney stone: a possible path to prophylaxis of nephrolithiasis? Focus on "Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells".
Heneghan JF, Alper SL. Heneghan JF, et al. Am J Physiol Cell Physiol. 2012 Jan 1;302(1):C18-20. doi: 10.1152/ajpcell.00389.2011. Epub 2011 Nov 2. Am J Physiol Cell Physiol. 2012. PMID: 22049207 No abstract available. - Re: net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion.
Assimos D. Assimos D. J Urol. 2012 May;187(5):1926-8. doi: 10.1016/j.juro.2012.01.038. Epub 2012 Mar 21. J Urol. 2012. PMID: 22494765 No abstract available.
Similar articles
- Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-δ activation.
Amin R, Sharma S, Ratakonda S, Hassan HA. Amin R, et al. Am J Physiol Cell Physiol. 2013 Jul 1;305(1):C78-89. doi: 10.1152/ajpcell.00339.2012. Epub 2013 Apr 17. Am J Physiol Cell Physiol. 2013. PMID: 23596171 Free PMC article. - Adenosinergic signaling inhibits oxalate transport by human intestinal Caco2-BBE cells through the A2B adenosine receptor.
Jung D, Alshaikh A, Ratakonda S, Bashir M, Amin R, Jeon S, Stevens J, Sharma S, Ahmed W, Musch M, Hassan H. Jung D, et al. Am J Physiol Cell Physiol. 2018 Nov 1;315(5):C687-C698. doi: 10.1152/ajpcell.00024.2017. Epub 2018 Jul 18. Am J Physiol Cell Physiol. 2018. PMID: 30020825 Free PMC article. - Regulation of anion exchanger Slc26a6 by protein kinase C.
Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS. Hassan HA, et al. Am J Physiol Cell Physiol. 2007 Apr;292(4):C1485-92. doi: 10.1152/ajpcell.00447.2006. Epub 2006 Dec 6. Am J Physiol Cell Physiol. 2007. PMID: 17151144 - Role of SLC26A6-mediated Cl⁻-oxalate exchange in renal physiology and pathophysiology.
Aronson PS. Aronson PS. J Nephrol. 2010 Nov-Dec;23 Suppl 16:S158-64. J Nephrol. 2010. PMID: 21170874 Review. - Essential roles of CFEX-mediated Cl(-)-oxalate exchange in proximal tubule NaCl transport and prevention of urolithiasis.
Aronson PS. Aronson PS. Kidney Int. 2006 Oct;70(7):1207-13. doi: 10.1038/sj.ki.5001741. Epub 2006 Aug 2. Kidney Int. 2006. PMID: 16883319 Review.
Cited by
- Oxalate as a potent promoter of kidney stone formation.
Chen T, Qian B, Zou J, Luo P, Zou J, Li W, Chen Q, Zheng L. Chen T, et al. Front Med (Lausanne). 2023 Jun 5;10:1159616. doi: 10.3389/fmed.2023.1159616. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37342493 Free PMC article. Review. - Sel1-like proteins and peptides are the major _Oxalobacter formigenes_-derived factors stimulating oxalate transport by human intestinal epithelial cells.
Arvans D, Chang C, Alshaikh A, Tesar C, Babnigg G, Wolfgeher D, Kron S, Antonopoulos D, Bashir M, Cham C, Musch M, Chang E, Joachimiak A, Hassan H. Arvans D, et al. Am J Physiol Cell Physiol. 2023 Jul 1;325(1):C344-C361. doi: 10.1152/ajpcell.00466.2021. Epub 2023 May 1. Am J Physiol Cell Physiol. 2023. PMID: 37125773 Free PMC article. - Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-δ activation.
Amin R, Sharma S, Ratakonda S, Hassan HA. Amin R, et al. Am J Physiol Cell Physiol. 2013 Jul 1;305(1):C78-89. doi: 10.1152/ajpcell.00339.2012. Epub 2013 Apr 17. Am J Physiol Cell Physiol. 2013. PMID: 23596171 Free PMC article. - Activation of the PKA signaling pathway stimulates oxalate transport by human intestinal Caco2-BBE cells.
Arvans D, Alshaikh A, Bashir M, Weber C, Hassan H. Arvans D, et al. Am J Physiol Cell Physiol. 2020 Feb 1;318(2):C372-C379. doi: 10.1152/ajpcell.00135.2019. Epub 2019 Dec 11. Am J Physiol Cell Physiol. 2020. PMID: 31825656 Free PMC article. - Kidney stones: an update on current pharmacological management and future directions.
Xu H, Zisman AL, Coe FL, Worcester EM. Xu H, et al. Expert Opin Pharmacother. 2013 Mar;14(4):435-47. doi: 10.1517/14656566.2013.775250. Expert Opin Pharmacother. 2013. PMID: 23438422 Free PMC article. Review.
References
- Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339–351, 2011 - PubMed
- Arpin M, Blair L, Coudrier E, Dudouet B, Finidori J, Carcia A, Huet C, Pringault E, Robine S, Sahuguillo-Merino C, Louvard D. Villin, a specific marker for some epithelia specialized in transport, to study the differentiation of intestinal and kidney cells in vivo and in a human colon adenocarcinoma line HT29 in culture. Mol Aspects Med 10: 257–272, 1988. - PubMed
- Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am 31: 927–949, 2002 - PubMed
- Bertrand CA, Laboisse CL, Hopfer U. Purinergic and cholinergic agonists induce exocytosis from the same granule pool in HT29-Cl.16E monolayers. Am J Physiol Cell Physiol 276: C907–C914, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37-DK-33793/DK/NIDDK NIH HHS/United States
- R37 DK033793/DK/NIDDK NIH HHS/United States
- K08-DK-067245/DK/NIDDK NIH HHS/United States
- K08 DK067245/DK/NIDDK NIH HHS/United States
- P01-DK-17433/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous