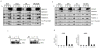Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation - PubMed (original) (raw)
Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation
Ryan G Holzer et al. Cell. 2011.
Abstract
Saturated fatty acids (FA) exert adverse health effects and are more likely to cause insulin resistance and type 2 diabetes than unsaturated FA, some of which exert protective and beneficial effects. Saturated FA, but not unsaturated FA, activate Jun N-terminal kinase (JNK), which has been linked to obesity and insulin resistance in mice and humans. However, it is unknown how saturated and unsaturated FA are discriminated. We now demonstrate that saturated FA activate JNK and inhibit insulin signaling through c-Src activation. FA alter the membrane distribution of c-Src, causing it to partition into intracellular membrane subdomains, where it likely becomes activated. Conversely, unsaturated FA with known beneficial effects on glucose metabolism prevent c-Src membrane partitioning and activation, which are dependent on its myristoylation, and block JNK activation. Consumption of a diabetogenic high-fat diet causes the partitioning and activation of c-Src within detergent insoluble membrane subdomains of murine adipocytes.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
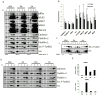
Figure 1. c-Src is required for JNK activation and insulin resistance by saturated FA
A–D. JNK activation by FAs is c-Src dependent. A. NIH3T3, Src−/−, SYF−/− and SYF fibroblasts reconstituted with c-Src were treated for 6 hrs with 500 μM palmitic (PA) or stearic (SA) acids loaded onto BSA. Whole cell lysates were prepared and subjected to JNK1 immunecomplex kinase assay using GST-c-Jun (1–79) as a substrate and immunoblotting with indicated antibodies. B. NIH3T3 and SYF−/− fibroblasts transduced with empty vector, Fyn or Yes expression vectors were treated with SA or PA and analyzed as above. C. NIH3T3 cells were infected with lentiviral constructs carrying scrambled (Scr) or c-Src-specific shRNAs. After selection in puromycin-containing medium, cells were treated with PA and JNK1 activity and c-Src expression were analyzed. D. NIH3T3, Src−/− cells and Src−/− cells transduced with empty vector or wt c-Src or c-Src(Y418F) expression vectors, or SYF−/− cells reconstituted with wt, Y527F or Y527F/G2A c-Src vectors were treated with PA-loaded BSA or BSA alone and analyzed as above. Fold-increase in JNK1 activity is shown below and was determined by densitometric analysis of 3 similar, but separate experiments. E. Src is required for MLK3 activation by PA. NIH3T3, SYF−/− and SYF+Src cells were treated with PA-loaded BSA for the indicated time periods. Cell lysates were prepared and phosphorylation of MLK3 and JNK1/2 was monitored by immunoblotting. F. PA-induced insulin resistance is c-Src dependent. NIH3T3, SYF−/− and SYF+Src cells were pretreated with or without PA for 6 hrs before treatment with 100 ng/ml insulin for 7.5 and 15 min. JNK activation and insulin-induced AKT phosphorylation were analyzed by immunoblotting.
Figure 2. Saturated FA induce c-Src segregation into detergent resistant membranes
A. SYF+Src cells were treated for 4 hrs with BSA alone or PA- or SA-loaded BSA. Whole-cell lysates were solubilized with Triton X100 at 4°C and DRMs were isolated by equilibrium density gradient centrifugation. Fractions were collected from the gradient’s top, such that fraction 1 represents the DRM fraction. Presence of the indicated proteins and phospho-Tyr418 c-Src in the different fractions was analyzed by immunoblotting. Flotillin-1 and flotillin-2 are lipid raft markers, whereas calnexin is a membrane protein that is excluded from lipid rafts/DRM. The percentage of c-Src in Fr. 1 is indicated underneath and was calculated by densitometric analysis of 4 separate experiments. B. FA-induced enrichment of different signaling proteins in the DRM fraction following PA or SA treatments. Results are averages ± s.d. of 3–5 experiments similar to the one in A (*: p < 0.05). C. c-Src kinase activity in different membrane fractions from cells treated with BSA or BSA plus saturated FA. Cells were treated and their membranes were fractionated as in A. c-Src was immunoprecipitated from the different fractions and its activity measured using acid-activated enolase as a substrate. Average fold-increase in c-Src activity (n=4 for PA and n=3 for SA) is indicated below. D. c-Src membrane redistribution is dependent on FA acyl chain length. SYF+Src cells were treated with 500 μM PA (C16), MA (C14) or LA (C12). The cells were fractionated as in A and the fractions were immunoblotted for the indicated proteins. E. SYF+Src cells were treated for 6 hrs with either BSA alone or BSA loaded with PA, MA, or LA. Induction of CHOP mRNA or XBP1 mRNA splicing was analyzed by Q-RT-PCR. Results are averages ± s.d. (n=3).
Figure 3. Mono- and poly-unsaturated FA block c-Src membrane redistribution and activation as well as JNK activation and induction of ER stress markers
A-D. Unsaturated FA inhibit c-Src segregation and activation within DRM in response to PA. SYF+Src cells were pretreated with either monounsaturated palmitoleic acid (POA) (A) or polyunsaturated eicosapentaenoic acid (EPA) (B) for 15 min prior to addition of BSA or BSA loaded with PA. After 4 hrs, membranes were solubilized and fractionated as in Figure 2 and distribution of the indicated proteins was determined by immunoblotting. c-Src and phospho-Tyr418 c-Src accumulated in the DRM fraction of PA-treated cells, and this was prevented by pretreatment with either POA (A) or EPA (B). (C) JNK activation and (D) induction of ER stress markers in the cells subjected to the above treatments was assessed as described in Figures 1 and 2.
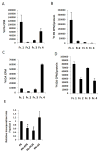
Figure 4. Saturated FA are preferentially incorporated into DRM
A, B. SYF+Src cells were treated for 2 hrs with 200 μM 3H-labeled PA and 300 μM cold PA loaded onto BSA. Cell lysates were prepared and membranes were solubilized and fractionated as in Figure 2. (A) The amount of 3H in each fraction was determined by scintillation counting and (B) normalized to protein content. Results are averages ± s.d. (n=3). C, D. SYF+Src cells were treated for 2 hrs with 110 nM 3H-labeled OA and 300 μM cold OA loaded onto BSA. Cells were fractionated as above and (C) The amount of 3H in each fraction was determined by scintillation counting and (D) normalized to protein content. Results are averages ± s.d. (n=3). E. SYF+Src cells were pretreated for 15 min with 300 μM EPA, POA or SA, followed by a 2 hr incubation with 200 μM 3H-labeled PA and 300 μM cold PA loaded onto BSA. Cell lysates were solubilized and fractionated and the relative amount of 3H in fraction 1 was determined as above. Results are averages ± s.d. (n=3; *: p < 0.05).
Figure 5. Consumption of high fat diet results in c-Src segregation and activation within DRM of adipose tissue
A–D. Mice were kept on normal chow (LFD) or high fat diet (HFD) for 16 weeks after which brown (A, B; BAT) and white (C, D; WAT) adipose tissues were isolated. The tissues were homogenized, excess lipid was removed and the lysates were adjusted to equal protein concentrations before cold Triton X100 solubilization and fractionation as in Figure 2. A. Density gradient fractions of BAT membranes were analyzed for the indicated proteins by immunoblotting. Results show two different mice for each dietary condition and are representative of separate experiments in which 3 mice were analyzed. B. c-Src was immunoprecipitated from the gradient fractions of BAT isolated from mice LFD2 and HFD2 and its kinase activity was measured using enolase as a substrate. C. Density gradient fractions of WAT membranes from the indicated mice were analyzed for the indicated proteins by immunoblotting. D. c-Src was immunoprecipitated from fraction 1 of 3 different LFD- or HFD-fed mice and its kinase activity was measured as above.
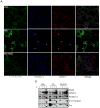
Figure 6. Subcellular localization and redistribution of activated c-Src in FA-treated fibroblasts
A. SYF+Src cells were treated for 2.5 hrs with BSA, PA or PA+POA before fixation and staining with antibodies to phospho-Tyr418 c-Src, LAMP-1 or flotillin-1. Three-color confocal images were acquired on a Leica SPE-2 confocal microscope. Magnification: 63x. Arrows indicate aggregates of phospho-Tyr418 c-Src that co-localize with flotillin-1 and LAMP-1. B. SYF+Src cells were treated as above. Cells were lysed in detergent-free buffer, and post-nuclear lysates were separated on a Percoll gradient under high speed centrifugation. A band enriched for LAMP-1 was collected, immediately resuspended in buffer and solubilized with Triton X100 at 4°C, and then fractionated on density gradients. Fractions were collected and examined for the indicated proteins by immunoblotting.
Comment in
- Long, saturated chains: tasty domains for kinases of insulin resistance.
Lizunov V, Chlanda P, Kraft M, Zimmerberg J. Lizunov V, et al. Dev Cell. 2011 Oct 18;21(4):604-6. doi: 10.1016/j.devcel.2011.10.001. Dev Cell. 2011. PMID: 22014518 Free PMC article.
Similar articles
- Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes.
Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Gao Z, et al. Mol Endocrinol. 2004 Aug;18(8):2024-34. doi: 10.1210/me.2003-0383. Epub 2004 May 13. Mol Endocrinol. 2004. PMID: 15143153 - MLK3 promotes metabolic dysfunction induced by saturated fatty acid-enriched diet.
Gadang V, Kohli R, Myronovych A, Hui DY, Perez-Tilve D, Jaeschke A. Gadang V, et al. Am J Physiol Endocrinol Metab. 2013 Aug 15;305(4):E549-56. doi: 10.1152/ajpendo.00197.2013. Epub 2013 Jul 16. Am J Physiol Endocrinol Metab. 2013. PMID: 23860122 Free PMC article. - Interplay of Dietary Fatty Acids and Cholesterol Impacts Brain Mitochondria and Insulin Action.
Schell M, Chudoba C, Leboucher A, Alfine E, Flore T, Ritter K, Weiper K, Wernitz A, Henkel J, Kleinridders A. Schell M, et al. Nutrients. 2020 May 23;12(5):1518. doi: 10.3390/nu12051518. Nutrients. 2020. PMID: 32456175 Free PMC article. - Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes.
Yung JHM, Giacca A. Yung JHM, et al. Cells. 2020 Mar 13;9(3):706. doi: 10.3390/cells9030706. Cells. 2020. PMID: 32183037 Free PMC article. Review. - Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function.
Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D, Soulage CO. Guebre-Egziabher F, et al. Biochimie. 2013 Nov;95(11):1971-9. doi: 10.1016/j.biochi.2013.07.017. Epub 2013 Jul 27. Biochimie. 2013. PMID: 23896376 Review.
Cited by
- A gp130-Src-YAP module links inflammation to epithelial regeneration.
Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, Sandborn WJ, Hardiman G, Raz E, Maehara Y, Yoshimura A, Zucman-Rossi J, Guan KL, Karin M. Taniguchi K, et al. Nature. 2015 Mar 5;519(7541):57-62. doi: 10.1038/nature14228. Epub 2015 Feb 25. Nature. 2015. PMID: 25731159 Free PMC article. - Inhibitory effects of omega-3 fatty acids on injury-induced epidermal growth factor receptor transactivation contribute to delayed wound healing.
Turk HF, Monk JM, Fan YY, Callaway ES, Weeks B, Chapkin RS. Turk HF, et al. Am J Physiol Cell Physiol. 2013 May 1;304(9):C905-17. doi: 10.1152/ajpcell.00379.2012. Epub 2013 Feb 20. Am J Physiol Cell Physiol. 2013. PMID: 23426968 Free PMC article. - Molecular Mechanisms of Apoptosis Induction and Its Regulation by Fatty Acids in Pancreatic β-Cells.
Šrámek J, Němcová-Fürstová V, Kovář J. Šrámek J, et al. Int J Mol Sci. 2021 Apr 20;22(8):4285. doi: 10.3390/ijms22084285. Int J Mol Sci. 2021. PMID: 33924206 Free PMC article. Review. - Granulocyte colony-stimulating factor (G-CSF): A saturated fatty acid-induced myokine with insulin-desensitizing properties in humans.
Ordelheide AM, Gommer N, Böhm A, Hermann C, Thielker I, Machicao F, Fritsche A, Stefan N, Häring HU, Staiger H. Ordelheide AM, et al. Mol Metab. 2016 Feb 13;5(4):305-316. doi: 10.1016/j.molmet.2016.02.001. eCollection 2016 Apr. Mol Metab. 2016. PMID: 27069870 Free PMC article. - Mechanisms of inflammatory responses in obese adipose tissue.
Sun S, Ji Y, Kersten S, Qi L. Sun S, et al. Annu Rev Nutr. 2012 Aug 21;32:261-86. doi: 10.1146/annurev-nutr-071811-150623. Epub 2012 Mar 9. Annu Rev Nutr. 2012. PMID: 22404118 Free PMC article. Review.
References
- Agostino N, Chinchilli VM, Lynch CJ, Koszyk-Szewczyk A, Gingrich R, Sivik J, Drabick JJ. Effect of the tyrosine kinase inhibitors (sunitinib, sorafenib, dasatinib, and imatinib) on blood glucose levels in diabetic and nondiabetic patients in general clinical practice. J Oncol Pharm Pract. 2010 Online publication. - PubMed
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. - PubMed
- Arcaro A, Aubert M, Espinosa del Hierro ME, Khanzada UK, Angelidou S, Tetley TD, Bittermann AG, Frame MC, Seckl MJ. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cell Signal. 2007;19:1081–1092. - PubMed
- Breccia M, Muscaritoli M, Cannella L, Stefanizzi C, Frustaci A, Alimena G. Fasting glucose improvement under dasatinib treatment in an accelerated phase chronic myeloid leukemia patient unresponsive to imatinib and nilotinib. Leuk Res. 2008;32:1626–1628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES0100337/ES/NIEHS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R37 ES004151/ES/NIEHS NIH HHS/United States
- ES006376/ES/NIEHS NIH HHS/United States
- R01 ES006376/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous