Upregulated expression of cytotoxicity-related genes in IFN-γ knockout mice with Schistosoma japonicum infection - PubMed (original) (raw)
Upregulated expression of cytotoxicity-related genes in IFN-γ knockout mice with Schistosoma japonicum infection
Xiaotang Du et al. J Biomed Biotechnol. 2011.
Abstract
It is well accepted that IFN-γ is important to the development of acquired resistance against murine schistosomiasis. However, the in vivo role of this immunoregulatory cytokine in helminth infection needs to be further investigated. In this study, parasite burden and host immune response were observed in IFN-γ knockout mice (IFNg KO) infected with Schistosoma japonicum for 6 weeks. The results suggested that deficiency in IFN-γ led to decreased egg burden in mice, with low schistosome-specific IgG antibody response and enhanced activation of T cells during acute infection. Microarray and qRT-PCR data analyses showed significant upregulation of some cytotoxicity-related genes, including those from the granzyme family, tumor necrosis factor, Fas Ligand, and chemokines, in the spleen cells of IFNg KO mice. Furthermore, CD8+ cells instead of NK cells of IFNg KO mice exhibited increased transcription of cytotoxic genes compared with WT mice. Additionally, Schistosoma japonicum-specific egg antigen immunization also could activate CD8+ T cells to upregulate the expression of cytotoxic genes in IFNg KO mice. Our data suggest that IFN-γ is not always a positive regulator of immune responses. In certain situations, the disruption of IFN-γ signaling may up-regulate the cytotoxic T-cell-mediated immune responses to the parasite.
Figures
Figure 1
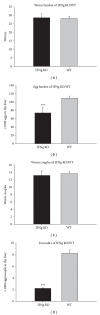
Parasite burden of IFNg KO mice and WT mice (n = 10, resp.) at 6 weeks after-infection with Schistosoma japonicum (compared with WT mice, **P < 0.01). (a) Total worms were recovered by portal perfusion at 6 weeks after-infection. (b) Eggs deposited in the liver were counted after digestion of the liver with 5% KOH. (c) Worm pairs were recovered by portal perfusion at 6 weeks after-infection. (d) Eggs deposited per worm couple in the liver. Data are representative of two independent experiments with the similar results.
Figure 2
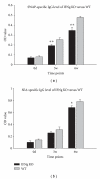
Dynamics of SWAP- and SEA-specific IgG antibody levels in IFNg KO and WT mice (n = 10, resp.) according to ELISA of sera harvested at day 0, 3 weeks, and 6 weeks after-infection (compared with WT mice, *P < 0.05, **P < 0.01). Data are representative of two independent experiments with the similar results.
Figure 3
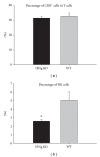
Percentage of CD8+ cells among T cells and NK cells among spleen cells as determined by FACS at 6 weeks after-infection with Schistosoma japonicum (compared with WT mice, *P < 0.05).
Figure 4
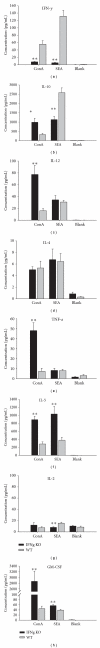
Type 1/Type 2 cytokine levels in the supernatant of splenocyte cultures of IFNg KO and WT mice (n = 10, resp.) at 6 weeks after Schistosoma japonicum infection by Bio-Plex detection (compared with WT mice, *P < 0.05, **P < 0.01). Data are representative of two independent experiments with the similar results.
Figure 5
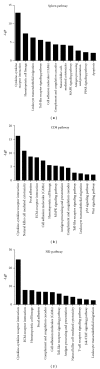
Significantly upregulated pathways with differentially expressed genes in splenocytes, purified CD8+ cells, and NK cells in 6-week _Schistosoma japonicum_-infected IFNg KO mice compared with WT mice based on KEGG and GENEMAP databases. The value of “-LgP” stands for the significance of a particular pathway category.
Figure 6
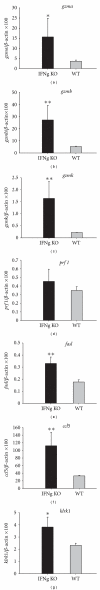
Relative transcription levels of gzma, gzmb, gzmk, prf1, fasl, ccl5, and klrk1 in splenocytes of _Schistosoma japonicum_-infected IFNg KO and WT mice by real-time PCR. Data were analyzed using the Mann-Whitney test for statistical support (compared with WT mice, *P < 0.05, **P < 0.01).
Figure 7
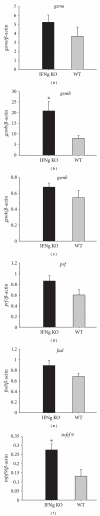
Expression of gzma, gzmb, gzmk, prf1, fasl and tnfsf9 in purified CD8+ cells from SEA-immunized mice, as measured by qRT-PCR (compared with WT mice, *P < 0.05, **P < 0.01).
Figure 8
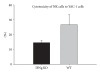
Cytotoxicity of NK cells exposed to YAC-1 cells from IFNg KO and WT mice immunized with SEA.
Similar articles
- Gene expression profile of CD4+ T cells reveals an interferon signaling suppression associated with progression of experimental Schistosoma japonicum infection.
Ji MJ, Su C, Wu HW, Zhu X, Cai XP, Li CL, Li GF, Wang Y, Zhang ZS, Wu GL. Ji MJ, et al. Cell Immunol. 2003 Jul;224(1):55-62. doi: 10.1016/j.cellimm.2003.08.001. Cell Immunol. 2003. PMID: 14572801 - TLR2 directing PD-L2 expression inhibit T cells response in Schistosoma japonicum infection.
Gao Y, Chen L, Hou M, Chen Y, Ji M, Wu H, Wu G. Gao Y, et al. PLoS One. 2013 Dec 20;8(12):e82480. doi: 10.1371/journal.pone.0082480. eCollection 2013. PLoS One. 2013. PMID: 24376539 Free PMC article. - Ikzf2 Regulates the Development of ICOS+ Th Cells to Mediate Immune Response in the Spleen of _S. japonicum_-Infected C57BL/6 Mice.
Xie S, Wei H, Peng A, Xie A, Li J, Fang C, Shi F, Yang Q, Huang H, Xie H, Pan X, Tian X, Huang J. Xie S, et al. Front Immunol. 2021 Aug 12;12:687919. doi: 10.3389/fimmu.2021.687919. eCollection 2021. Front Immunol. 2021. PMID: 34475870 Free PMC article. - The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion.
Mojic M, Takeda K, Hayakawa Y. Mojic M, et al. Int J Mol Sci. 2017 Dec 28;19(1):89. doi: 10.3390/ijms19010089. Int J Mol Sci. 2017. PMID: 29283429 Free PMC article. Review.
Cited by
- Immune modulation of Th1/Th2/Treg/Th17/Th9/Th21 cells in rabbits infected with Eimeria stiedai.
Chen XD, Xie J, Wei Y, Yu JF, Cao Y, Xiao L, Wu XJ, Mao CJ, Kang RM, Ye YG. Chen XD, et al. Front Cell Infect Microbiol. 2023 Aug 1;13:1230689. doi: 10.3389/fcimb.2023.1230689. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37593762 Free PMC article. - Immune modulation of Th1, Th2, and T-reg transcriptional factors differing from cytokine levels in Schistosoma japonicum infection.
Farwa A, He C, Xia L, Zhou H. Farwa A, et al. Parasitol Res. 2018 Jan;117(1):115-126. doi: 10.1007/s00436-017-5678-5. Epub 2017 Nov 29. Parasitol Res. 2018. PMID: 29188369 - The M1 and M2 paradigm of macrophage activation: time for reassessment.
Martinez FO, Gordon S. Martinez FO, et al. F1000Prime Rep. 2014 Mar 3;6:13. doi: 10.12703/P6-13. eCollection 2014. F1000Prime Rep. 2014. PMID: 24669294 Free PMC article. Review. - Mice lack of LRG-47 display the attenuated outcome of infection with Schistosoma japonicum.
Gao Y, Wu J, Zhang M, Hou M, Ji M. Gao Y, et al. Parasitol Res. 2016 Mar;115(3):1185-93. doi: 10.1007/s00436-015-4853-9. Epub 2015 Dec 10. Parasitol Res. 2016. PMID: 26660918
References
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annual Review of Immunology. 1997;15:749–795. - PubMed
- Hewitson JP, Hamblin PA, Mountford AP. Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunology. 2005;27(7-8):271–280. - PubMed
- Kariuki TM, Farah IO. Resistance to re-infection after exposure to normal and attenuated schistosome parasites in the baboon model. Parasite Immunology. 2005;27(7-8):281–288. - PubMed
- Bickle QD, Bogh HO, Johansen MV, Zhang Y. Comparison of the vaccine efficacy of γ-irradiated Schistosoma japonicum cercariae with the defined antigen Sj62(IrV-5) in pigs. Veterinary Parasitology. 2001;100(1-2):51–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous