Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity - PubMed (original) (raw)
. 2011 Oct 15;25(20):2125-36.
doi: 10.1101/gad.17276711. Epub 2011 Oct 6.
Claudio Scuoppo, Xiaowo Wang, Xueping Fang, Brian Balgley, Jessica E Bolden, Prem Premsrirut, Weijun Luo, Agustin Chicas, Cheng S Lee, Scott C Kogan, Scott W Lowe
Affiliations
- PMID: 21979375
- PMCID: PMC3205583
- DOI: 10.1101/gad.17276711
Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity
Yuchen Chien et al. Genes Dev. 2011.
Abstract
Cellular senescence acts as a potent barrier to tumorigenesis and contributes to the anti-tumor activity of certain chemotherapeutic agents. Senescent cells undergo a stable cell cycle arrest controlled by RB and p53 and, in addition, display a senescence-associated secretory phenotype (SASP) involving the production of factors that reinforce the senescence arrest, alter the microenvironment, and trigger immune surveillance of the senescent cells. Through a proteomics analysis of senescent chromatin, we identified the nuclear factor-κB (NF-κB) subunit p65 as a major transcription factor that accumulates on chromatin of senescent cells. We found that NF-κB acts as a master regulator of the SASP, influencing the expression of more genes than RB and p53 combined. In cultured fibroblasts, NF-κB suppression causes escape from immune recognition by natural killer (NK) cells and cooperates with p53 inactivation to bypass senescence. In a mouse lymphoma model, NF-κB inhibition bypasses treatment-induced senescence, producing drug resistance, early relapse, and reduced survival. Our results demonstrate that NF-κB controls both cell-autonomous and non-cell-autonomous aspects of the senescence program and identify a tumor-suppressive function of NF-κB that contributes to the outcome of cancer therapy.
Figures
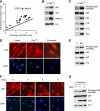
Figure 1.
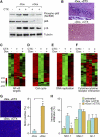
NF-κB is activated in senescent cells. (A) A scatter plot of 18 transcription factors identified from mass spectrometry (refer to Supplemental Table 1 for the full list of proteins identified). The line represents values of y = x, which means the expression is the same for growing and senescent cells. (B) Increased binding of p65 to senescent chromatin fraction. Chromatin fractions were isolated from growing (containing empty vector, abbreviated as V) and senescent IMR-90 cells induced by H-RasV12 and analyzed by Western blotting using the indicated antibodies. H3 was used as loading control. (C) Hyperphosphorylation of p65 on Ser 536 in senescent cells. Whole-cell lysates from growing and H-RasV12-induced senescent IMR-90 cells were analyzed by Western blotting using the indicated antibodies. Accumulation of p53, p16INK4a, and p15INK4b proteins are shown as senescence markers. Total p65 was used as loading control. (D) Senescent cells show nuclear translocation and accumulation of p65. Growing and senescent IMR-90 cells induced by either H-RasV12 or etoposide (100 μM) were immunostained with anti-p65 antibodies (top panels) and counterstained with DAPI (bottom panels) to show SAHF formation. (E) Whole-cell lysates from growing and etoposide-induced senescent IMR-90 cells were analyzed by Western blotting using the indicated antibodies. (F) Nuclear translocation of p65 coincides with senescence. IMR-90 cells containing RasV12-ER were treated with 4-OHT (200 μM) for the indicated time. Cells were immunostained with anti-p65 antibodies (top panels) and counterstained with DAPI (bottom panels). (G) Whole-cell lysates from cells in parallel with conditions described in F were collected and analyzed by Western blotting using the indicated antibodies.
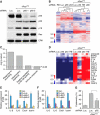
Figure 2.
NF-κB mediates the SASP. (A) IMR-90 cells were infected with the indicated shRNAs with or without H-RasV12. shRNA against firefly luciferase (Luc.) was used as a control. Whole-cell lysates were collected 8 d following the completion of selection and analyzed by Western blotting using the indicated antibodies. Total ERK was used as loading control. (B) Hierarchical clustering analysis of differentially expressed genes from IMR-90 cells expressing each indicated shRNA with and without H-RasV12. The shRNA against p65 that showed the best knockdown efficiency by Western blotting (shp65-1) was used. (C) GO analysis of significantly down-regulated genes upon loss of p65 function (P < 0.05). Differentially expressed genes were identified by comparing samples with shRNA against p65 with that of luciferase in H-RasV12-induced senescent IMR-90 cells. (D) Heat map of genes comprising the GO term “immune response.” Loss of p65 significantly down-regulated immune response genes (P = 3.7 × 10−2 with Benjamini correction). (E) Suppression of p65 function abrogates the induction of IL-6, IL-8, CXCL1, and ICAM-1 in senescent cells. Total RNA was extracted and analyzed by RT-qPCR with primer sets against the indicated transcripts. Expression is normalized to the expression of β-actin and is expressed as the fold change relative to growing IMR-90 cells. Values are means + SEM of two independent samples with triplicate qPCR. (Vec.) Vector; (R.) H-RasV12; (Luc.) shRNA against luciferase; (p65-1 and p65-2) two shRNAs against p65. (F) NF-κB inhibitor BAY11-7082 (BAY) also prevents the induction of IL-6, IL-8, CXCL1, and ICAM-1 in senescent cells. IMR-90 cells were infected with the indicated shRNAs with or without H-RasV12. Eight days following the completion of selection, senescent IMR-90 cells were treated with 10 μM BAY11-7082 for 2 d before harvesting. Expression is normalized to the expression of β-actin and is expressed as the fold change relative to growing cells (containing vector). Values are means + SEM of two independent samples with triplicate qPCRs. (G) NF-κB is required for recognition and killing by NK cells. IMR-90 cells expressing the indicated vectors were cocultured with YT cells (a human NK cell line) for 12 h. Attached viable senescent cells were stained with crystal violet. Crystal violet quantification at OD 595 was performed and the percentage cytotoxicity was calculated as crystal violet absorbance in the presence of YT cells relative to senescent cells cultured in the absence of YT cells. Values represent means + SEM of three independent experiments; (*) P < 0.05 using Student's _t_-test.
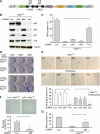
Figure 3.
NF-κB cooperates with p53 to promote senescence. (A) A schematic diagram of the polycistronic shRNA construct used to simultaneously knock down p65 and p53. (Tan) Tandem hairpin. The shRNA against p65 that showed the best knockdown efficiency by Western blotting (shp65-1) was used. (B) Coinhibition of p53 and p65 expression. IMR-90 cells were infected with the indicated shRNAs and H-RasV12. Whole-cell lysates were collected 8 d post-selection and analyzed by Western blotting using the indicated antibodies. Tubulin is shown as a loading control. (C) Coinhibition of p53 and p65 bypasses senescence. IMR-90 cells infected with the indicated shRNAs with or without H-RasV12 were analyzed for BrdU incorporation 8 d post-selection. Values represent means + SEM of two independent experiments; (*) P < 0.005 using Student's _t_-test. (D) IMR-90 cells infected with the indicated shRNAs and H-RasV12 were plated at increasing cell density 8 d post-selection. Cells were cultured for an additional 2 wk, stained with crystal violet, and analyzed for colony formation. (E) IMR-90 cells were infected with the indicated shRNAs and triggered to undergo senescence by either H-RasV12 or 100 μM etoposide. Eight days post-selection, cells were assayed for SA-β-gal activity. Values represent means + SEM of three independent experiments; (*) P < 0.05 using Student's _t_-test. (F) BJ cells were infected with the indicated shRNAs together with H-RasV12 to trigger senescence. Cells were assayed for SA-β-gal activity 8 d post-selection. Values represent means + SEM of three independent experiments; (*) P < 0.05 using Student's _t_-test. (G) BJ cells were infected with the indicated shRNAs with or without H-RasV12. Cells were assayed for BrdU incorporation 8 d post-selection. Values represent means + SEM of three independent experiments; (*) P < 0.05 using Student's _t_-test.
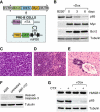
Figure 4.
Generation of Myc-Bcl-2-driven lymphomas with a tetracycline-regulatable p65 shRNA. (A) Bone marrow from 4- to 5-wk-old female mice carrying the Rosa-rtTA and the TRE-shRNA were infected with a vector coexpressing Myc and Bcl-2. Cells were explanted into pre-B-cell culture medium containing IL-7 for 1 wk. (B) Efficient knockdown of p65 after 6 d of Dox treatment. Cells from A were treated with or without Dox (1 μg/mL) for the indicated time, and whole-cell lysates were collected and analyzed by Western blotting using the indicated antibodies. Sorted B220+ cells from wild-type animals were used as a control. (C–E) Lymphoblastic neoplasms in recipient animals. Hematoxylin and eosin staining of lymph node (C), spleen (D), and liver (E) shows lymphoblastic disease. (W) Splenic white pulp; (R) splenic red pulp; lymphoma infiltrates both. (F) Myc- and Bcl-2-driven pre-B cells are resistant to apoptosis induced by chemotherapeutic agents. Cells from A were left untreated or treated with either ADR (250 ng/mL) or ABT-737 (10 μM) for 24 h in vitro. Whole-cell lysates were collected and analyzed by Western blotting using the indicated antibodies. (G) Treatment with chemotherapeutic agents does not engage necrosis in vivo. On and off Dox lymphoma-bearing mice were left untreated or treated with a single 200-μL dose of CTX (30 mg/mL). After 7 d, lymph nodes were harvested, prepared for single-cell suspension, sorted for CD43− B cells, and analyzed by Western blotting using the indicated antibodies.
Figure 5.
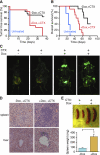
NF-κB inhibition compromises drug-induced senescence. (A) Activation of NF-κB occurs in chemotherapy-induced senescent lymphomas. Lymphoma-bearing mice with or without Dox treatment were injected with a single 200-μL dose of CTX (30 mg/mL) (n = 2 for each condition, except the CTX-untreated control). Lymph nodes from these mice were harvested and sorted for CD43− B cells, and whole-cell lysates were prepared and analyzed by Western blotting using the indicated antibodies. (B) Loss of p65 function bypasses drug-induced senescence. Seven days after CTX injection, lymph nodes from off Dox (p65-expressing) and on Dox (p65 knockdown) mice were harvested, cryo-sectioned, analyzed for SA-β-gal activity, and counterstained with eosin. (C–F) Heat map of differentially expressed NF-κB targets (C), cell cycle (D), DNA replication (E), and cytokine–cytokine receptor interaction genes (F) in each indicated condition (refer to Supplemental Table 3 for the full list of genes). (G) Inhibiting p65 function promotes cell growth after CTX treatment. Seven days after CTX injection, off Dox (p65-expressing) and on Dox (p65 knockdown) mice were injected intraperitoneally (i.p.) with 200 μL of BrdU solution (10 mg/mL) and sacrificed 2 h later. Lymph nodes from these mice were harvested, cryo-sectioned, immunostained for BrdU (red), and counterstained with DAPI (blue). Values represent means + SEM of at least three independent animals; (*) P < 0.05 using Student's _t_-test. (H) Loss of p65 function abrogates NK cell infiltration. Seven days after CTX injection, lymph nodes from off Dox (p65-expressing) and on Dox (p65 knockdown) mice were harvested, and single-cell suspensions were prepared and analyzed for the presence of specific immune cell types by flow cytometry. Percentages of total CD45.2+ cells are indicated. Values represent means + SEM of at least three independent animals; (*) P < 0.05 using Student's _t_-test.
Figure 6.
NF-κB inhibition leads to chemoresistance and poor survival in murine lymphomas. (A) Loss of p65 function results in chemoresistant lymphomas. Kaplan-Meier analysis of tumor-free survival as measured by lymphoma palpability following CTX therapy. Day 0 equals the day of CTX treatment. Curves reflect the responsiveness of off Dox (p65-expressing) (black line, n = 15) and on Dox (p65 knockdown) (red line, n = 15) lymphoma-bearing mice to CTX therapy. (B) Loss of p65 function leads to poor prognosis following chemotherapy. Kaplan-Meier survival analysis of on/off Dox lymphoma-bearing mice following CTX therapy (n = 10 for each group). Day 0 equals the day of CTX treatment. (C) Lymphoma-bearing mice with or without Dox treatment were sacrificed 3 wk after CTX injection and imaged for GFP. While lymphomas in off Dox mice (p65-expressing) regressed to undetectable levels, lymphomas in on Dox mice (p65 knockdown) persisted. (D) Suppression of p65 function leads to chemoresistant lymphoblastic neoplasms. Hematoxylin and eosin staining of spleens and livers from the on/off Dox diseased animals after CTX injection. Mice on Dox (p65 knockdown) showed a much more aggressive lymphoblastic disease. (E) Spleens from the on/off Dox diseased animals after CTX injection were dissected and weighed. Values represent means + SEM from at least four independent animals per group; (*) P < 0.05 using Student's _t_-test.
Comment in
- Senescence. NF-κB shows its beneficial side.
Burgess DJ. Burgess DJ. Nat Rev Cancer. 2011 Oct 28;11(12):832-3. doi: 10.1038/nrc3168. Nat Rev Cancer. 2011. PMID: 22020169 No abstract available.
Similar articles
- A previously identified apoptosis inhibitor iASPP confers resistance to chemotherapeutic drugs by suppressing senescence in cancer cells.
Li H, Zhang W, Zhao K, Zhao D, Zheng S, Hu Y. Li H, et al. J Biol Chem. 2020 Mar 20;295(12):4049-4063. doi: 10.1074/jbc.RA119.011411. Epub 2020 Jan 31. J Biol Chem. 2020. PMID: 32005663 Free PMC article. - Insight into the role of PIKK family members and NF-кB in DNAdamage-induced senescence and senescence-associated secretory phenotype of colon cancer cells.
Strzeszewska A, Alster O, Mosieniak G, Ciolko A, Sikora E. Strzeszewska A, et al. Cell Death Dis. 2018 Jan 19;9(2):44. doi: 10.1038/s41419-017-0069-5. Cell Death Dis. 2018. PMID: 29352261 Free PMC article. - Membrane-Bound CD40L Promotes Senescence and Initiates Senescence-Associated Secretory Phenotype via NF-κB Activation in Lung Adenocarcinoma.
Xu W, Li Y, Yuan WW, Yin Y, Song WW, Wang Y, Huang QQ, Zhao WH, Wu JQ. Xu W, et al. Cell Physiol Biochem. 2018;48(4):1793-1803. doi: 10.1159/000492352. Epub 2018 Aug 3. Cell Physiol Biochem. 2018. PMID: 30078020 - Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP).
Salminen A, Kauppinen A, Kaarniranta K. Salminen A, et al. Cell Signal. 2012 Apr;24(4):835-45. doi: 10.1016/j.cellsig.2011.12.006. Epub 2011 Dec 11. Cell Signal. 2012. PMID: 22182507 Review. - Regulation of senescence and the SASP by the transcription factor C/EBPβ.
Salotti J, Johnson PF. Salotti J, et al. Exp Gerontol. 2019 Dec;128:110752. doi: 10.1016/j.exger.2019.110752. Epub 2019 Oct 22. Exp Gerontol. 2019. PMID: 31648009 Review.
Cited by
- Body Composition and Senescence: Impact of Polyphenols on Aging-Associated Events.
Santos TWD, Pereira QC, Fortunato IM, Oliveira FS, Alvarez MC, Ribeiro ML. Santos TWD, et al. Nutrients. 2024 Oct 25;16(21):3621. doi: 10.3390/nu16213621. Nutrients. 2024. PMID: 39519454 Free PMC article. Review. - The role of the dynamic epigenetic landscape in senescence: orchestrating SASP expression.
Dasgupta N, Arnold R, Equey A, Gandhi A, Adams PD. Dasgupta N, et al. NPJ Aging. 2024 Oct 24;10(1):48. doi: 10.1038/s41514-024-00172-2. NPJ Aging. 2024. PMID: 39448585 Free PMC article. Review. - The Neolignan Honokiol and Its Synthetic Derivative Honokiol Hexafluoro Reduce Neuroinflammation and Cellular Senescence in Microglia Cells.
Sasia C, Borgonetti V, Mancini C, Lori G, Arbiser JL, Taddei ML, Galeotti N. Sasia C, et al. Cells. 2024 Oct 4;13(19):1652. doi: 10.3390/cells13191652. Cells. 2024. PMID: 39404415 Free PMC article. - Transcriptional repression by HDAC3 mediates T cell exclusion from Kras mutant lung tumors.
McGuire CK, Meehan AS, Couser E, Bull L, Minor AC, Kuhlmann-Hogan A, Kaech SM, Shaw RJ, Eichner LJ. McGuire CK, et al. Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2317694121. doi: 10.1073/pnas.2317694121. Epub 2024 Oct 10. Proc Natl Acad Sci U S A. 2024. PMID: 39388266 - A new model and precious tool to study molecular mechanisms of macrophage aging.
Smith R, Bassand K, Dussol A, Piesse C, Duplus E, El Hadri K. Smith R, et al. Aging (Albany NY). 2024 Oct 3;16(19):12697-12725. doi: 10.18632/aging.206124. Epub 2024 Oct 3. Aging (Albany NY). 2024. PMID: 39373702 Free PMC article.
References
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018 - PubMed
- Basseres DS, Baldwin AS 2006. Nuclear factor-κB and inhibitor of κB kinase pathways in oncogenic initiation and progression. Oncogene 25: 6817–6830 - PubMed
- Campisi J 2005. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120: 513–522 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R43 RR021239/RR/NCRR NIH HHS/United States
- T32 GM008444/GM/NIGMS NIH HHS/United States
- R21 CA143177/CA/NCI NIH HHS/United States
- RR021239/RR/NCRR NIH HHS/United States
- R01 AG016379/AG/NIA NIH HHS/United States
- R41 CA122715/CA/NCI NIH HHS/United States
- CA122715/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R42 CA122715/CA/NCI NIH HHS/United States
- CA143177/CA/NCI NIH HHS/United States
- AG16379/AG/NIA NIH HHS/United States
- R44 RR021239/RR/NCRR NIH HHS/United States
- R56 AG016379/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous