Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3β (GSK-3β)/β-catenin signaling cascade - PubMed (original) (raw)
Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3β (GSK-3β)/β-catenin signaling cascade
Di Zhang et al. J Biol Chem. 2011.
Abstract
Adiponectin is the most abundant adipokine secreted from adipocytes. Accumulating evidence suggests that the physiological roles of adiponectin go beyond its metabolic effects. In the present study, we demonstrate that adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) are expressed in adult hippocampal neural stem/progenitor cells (hNSCs). Adiponectin treatment increases proliferation of cultured adult hNSCs in a dose- and time-dependent manner, whereas apoptosis and differentiation of adult hNSCs into neuronal or glial lineage were not affected. Adiponectin activates AMP-activated protein kinase and p38 mitogen-activated protein kinase (p38MAPK) signaling pathways in adult hNSCs. Pretreatment with the p38MAPK inhibitor SB203580, but not the AMP-activated protein kinase inhibitor Compound C, attenuates adiponectin-induced cell proliferation. Moreover, adiponectin induces phosphorylation of Ser-389, a key inhibitory site of glycogen synthase kinase 3β (GSK-3β), and this effect can be blocked by inhibition of p38MAPK with SB203580. Levels of total and nuclear β-catenin, the primary substrate of GSK-3β, were increased by adiponectin treatment. These results indicate that adiponectin stimulates proliferation of adult hNSCs, via acting on GSK-3β to promote nuclear accumulation of β-catenin. Thus, our studies uncover a novel role for adiponectin signaling in regulating proliferation of adult neural stem cells.
Figures
FIGURE 1.
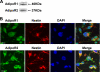
Expression of AdipoR1 and AdipoR2 in cultured adult hippocampal neural stem/progenitor cells. A, Western blot demonstrating the presence of AdipoR1 (46 kDa, top) and AdipoR2 (37 kDa, bottom) in cultured adult hippocampal neural stem/progenitor cells. B, immunocytochemical staining showing the colocalization of AdipoR1 (green, top) and AdipoR2 (green, bottom) with nestin (red), a neural stem cell marker. DAPI (blue) reveals nuclear counterstaining. Scale bar, 10 μm.
FIGURE 2.
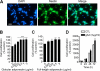
Effects of adiponectin on proliferation of adult hippocampal neural stem/progenitor cells. A, immunocytochemistry showing that cells are positive for the neural stem cell marker nestin (red). DAPI (blue) reveals nuclei. Scale bar, 20 μm. B and C, cells were treated with various doses of globular adiponectin (B) or full-length adiponectin (C) for 48 h. D, cells were treated with 3 μg/ml globular adiponectin (gAd) for 24, 48, or 72 h. Cell proliferation was determined by the MTT assay. Data are expressed as mean ± S.E. (n = 6 per group for B and n = 3 per group for C and D). *, p < 0.01; **, p < 0.001, ***, p < 0.0001, compared with vehicle controls. Error bars, S.E.
FIGURE 3.
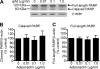
Effect of adiponectin on PARP cleavage in cultured adult hippocampal neural stem/progenitor cells. Adult hippocaampal neural stem/progenitor cells were incubated with various doses of globular adiponectin (gAd) for 48 h. Cleavage of PARP precursor protein was determined by Western blot analysis. A, representative immunoblots of full-length and cleaved PARP. B, quantitative data showing the effect of adiponectin on cleaved PARP. C, quantitative data showing the effect of adiponectin on full-length PARP. Data are expressed as mean ± S.E. (error bars), n = 4 per group.
FIGURE 4.
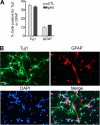
Effect of adiponectin on adult hippocampal neural stem/progenitor cell differentiation. A, cells were incubated with globular adiponectin (gAd; 3 μg/ml) and treated with 1 μ
m
retinoic acid and 0.5% FBS in culture medium for 6 days. The differentiation into neuronal or glial lineage was assessed by examining Tuj1, a neuronal marker, or GFAP, an astrocyte marker. Data are expressed as mean ± S.E. (error bars), n = 3 per group. B, representative images showing immunocytochemical staining for Tuj1 and GFAP and DAPI nuclear counterstain. Scale bar, 40 μm.
FIGURE 5.
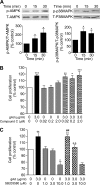
Effect of inhibition of AMPK and p38MAPK on adiponectin-induced proliferation of adult hippocampal neural stem/progenitor cells. A, effect of adiponectin on phosphorylation of AMPK (left) and p38MAPK (right) in adult hippocampal neural stem/progenitor cells at different time points (0, 15, and 30 min). Data are expressed as mean ± S.E. (error bars), n = 4 per group for AMPK, n = 3 per group for p38MAPK. *, p < 0.05; **, p < 0.01 compared with control. B, effect of inhibition of AMPK on adiponectin-induced cell proliferation. Cells were incubated with various concentrations of Compound C (0.02–2.0 μ
m
) for 2 h, followed by treatment with globular adiponectin (gAd; 3 μg/ml) for 48 h. Cell proliferation was assessed by MTT assay. C, effect of inhibition of p38MAPK on adiponectin-induced cell proliferation. Cells were incubated with various concentrations of SB203580 (1–10 μ
m
) for 2 h followed by treatment with globular adiponectin (3 μg/ml) for 48 h. Data in B and C are expressed as mean ± S.E., n = 6 per group. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with vehicle-vehicle control; ##, p < 0.01 compared with the vehicle plus 1.0 μ
m
SB203580 treatment; ++, p = 0.01 compared with the adiponectin-vehicle treatment.
FIGURE 6.
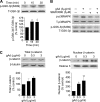
Effect of adiponectin on Ser-389 phosphorylation of GSK-3β in cultured adult hippocampal neural stem/progenitor cells. A, cells were incubated with 3 μg/ml globular adiponectin (gAd) for different time periods followed by immunoblotting with anti-phosphorylated Ser-389 (S389) of GSK-3β and anti-GSK-3β antibodies. Data are expressed as mean ± S.E. (error bars), n = 3 per group. *, p < 0.05 compared with control. B, cells were pretreated with a 3.0 μ
m
concentration of the p38MAPK inhibitor SB203580 at 2 h prior to adiponectin treatment (3.0 μg/ml) for 30 min. Representative immunoblots showing inhibition of phosphorylation of p38MAPK and Ser-389 phosphorylation of GSK-3β by SB203580. Similar results were obtained from two independent experiments. C, levels of β-catenin following adiponectin treatment for 48 h. Left, whole cell level of β-catenin. Right, nuclear level of β-catenin. Data are expressed as mean ± S.E., n = 3–4 per group. *, p < 0.05; **, p < 0.01 compared with vehicle control.
FIGURE 7.
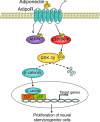
Signaling pathways possibly involved in adiponectin-induced proliferation of adult hippocampal neural stem/progenitor cells. Adiponectin activates p38MAPK, which phosphorylates GSK-3β on Ser-389, leading to inhibition of GSK-3β activity. This effect in turn results in reduced degradation of its substrate β-catenin and causes an accumulation of β-catenin in the nucleus, where it interacts with members of the lymphoid enhancer factor/T-cell factor (LEF/TCF) family of transcription factors and stimulates transcription of target genes, promoting neural stem/progenitor cell proliferation.
Similar articles
- Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice.
Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, Wu D, Cooper GJ, Xu A. Wang Y, et al. Cancer Res. 2006 Dec 1;66(23):11462-70. doi: 10.1158/0008-5472.CAN-06-1969. Cancer Res. 2006. PMID: 17145894 - Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3β/β-catenin signaling.
Garza JC, Guo M, Zhang W, Lu XY. Garza JC, et al. Mol Psychiatry. 2012 Jul;17(8):790-808. doi: 10.1038/mp.2011.161. Epub 2011 Dec 20. Mol Psychiatry. 2012. PMID: 22182938 Free PMC article. - Fluoxetine regulates neurogenesis in vitro through modulation of GSK-3β/β-catenin signaling.
Hui J, Zhang J, Kim H, Tong C, Ying Q, Li Z, Mao X, Shi G, Yan J, Zhang Z, Xi G. Hui J, et al. Int J Neuropsychopharmacol. 2014 Dec 7;18(5):pyu099. doi: 10.1093/ijnp/pyu099. Int J Neuropsychopharmacol. 2014. PMID: 25522429 Free PMC article. - Platelet-derived growth factor-BB restores HIV Tat -mediated impairment of neurogenesis: role of GSK-3β/β-catenin.
Chao J, Yang L, Yao H, Buch S. Chao J, et al. J Neuroimmune Pharmacol. 2014 Mar;9(2):259-68. doi: 10.1007/s11481-013-9509-x. Epub 2013 Nov 19. J Neuroimmune Pharmacol. 2014. PMID: 24248537 Free PMC article. - Glucocorticoids and lithium reciprocally regulate the proliferation of adult dentate gyrus-derived neural precursor cells through GSK-3beta and beta-catenin/TCF pathway.
Boku S, Nakagawa S, Masuda T, Nishikawa H, Kato A, Kitaichi Y, Inoue T, Koyama T. Boku S, et al. Neuropsychopharmacology. 2009 Feb;34(3):805-15. doi: 10.1038/npp.2008.198. Epub 2008 Nov 12. Neuropsychopharmacology. 2009. PMID: 19005466
Cited by
- Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors.
Zhang D, Wang X, Wang B, Garza JC, Fang X, Wang J, Scherer PE, Brenner R, Zhang W, Lu XY. Zhang D, et al. Mol Psychiatry. 2017 Jul;22(7):1044-1055. doi: 10.1038/mp.2016.58. Epub 2016 May 3. Mol Psychiatry. 2017. PMID: 27137743 Free PMC article. - Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity.
Liu J, Guo M, Zhang D, Cheng SY, Liu M, Ding J, Scherer PE, Liu F, Lu XY. Liu J, et al. Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):12248-53. doi: 10.1073/pnas.1202835109. Epub 2012 Jul 9. Proc Natl Acad Sci U S A. 2012. PMID: 22778410 Free PMC article. - Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study.
Song J, Kang SM, Kim E, Kim CH, Song HT, Lee JE. Song J, et al. Cell Death Dis. 2015 Aug 6;6(8):e1844. doi: 10.1038/cddis.2015.220. Cell Death Dis. 2015. PMID: 26247729 Free PMC article. - SB203580 reverses memory deficits and depression-like behavior induced by microinjection of Aβ1-42 into hippocampus of mice.
Guo J, Chang L, Li C, Li M, Yan P, Guo Z, Wang C, Zha Q, Wang Q. Guo J, et al. Metab Brain Dis. 2017 Feb;32(1):57-68. doi: 10.1007/s11011-016-9880-4. Epub 2016 Aug 3. Metab Brain Dis. 2017. PMID: 27488110 - The apoptotic volume decrease is an upstream event of MAP kinase activation during Staurosporine-induced apoptosis in HeLa cells.
Hasegawa Y, Shimizu T, Takahashi N, Okada Y. Hasegawa Y, et al. Int J Mol Sci. 2012;13(7):9363-9379. doi: 10.3390/ijms13079363. Epub 2012 Jul 24. Int J Mol Sci. 2012. PMID: 22942770 Free PMC article.
References
- Scherer P. E., Williams S., Fogliano M., Baldini G., Lodish H. F. (1995) J. Biol. Chem. 270, 26746–26749 - PubMed
- Maeda K., Okubo K., Shimomura I., Funahashi T., Matsuzawa Y., Matsubara K. (1996) Biochem. Biophys. Res. Commun. 221, 286–289 - PubMed
- Waki H., Yamauchi T., Kamon J., Kita S., Ito Y., Hada Y., Uchida S., Tsuchida A., Takekawa S., Kadowaki T. (2005) Endocrinology 146, 790–796 - PubMed
- Tsao T. S., Tomas E., Murrey H. E., Hug C., Lee D. H., Ruderman N. B., Heuser J. E., Lodish H. F. (2003) J. Biol. Chem. 278, 50810–50817 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous