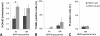p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model - PubMed (original) (raw)
p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model
Behrooz Azizi et al. Invest Ophthalmol Vis Sci. 2011.
Abstract
Purpose: This study compared susceptibility of Fuchs endothelial corneal dystrophy (FECD) and normal corneal endothelial cells (CECs) to oxidative stress, and studied the mechanism of oxidative-stress-induced apoptosis in FECD-affected endothelium.
Methods: For in vitro studies, immortalized normal and FECD human corneal endothelial cell lines (HCECi and FECDi, respectively) were exposed to tert-butyl hydroperoxide (tBHP). Apoptotic cell populations were distinguished using flow cytometry. Reactive oxygen species production was measured by a horseradish peroxidase assay. For ex vivo studies, CECs were exposed to tBHP. Oxidative DNA damage and apoptosis were assessed by anti-8-hydroxydeoxyguanosine antibody and TUNEL assay, respectively. p53 and phospho-p53 levels were assessed by Western blot and immunohistochemistry.
Results: Flow cytometry revealed a higher rate of apoptosis in FECDi than that in HCECi after exposure to 0.5 mM (P=0.010) and 1.0 mM tBHP (P=0.041). Further analysis showed increased production of H2O2 by FECDi than that by HCECi. Oxidative DNA damage increased in both normal and FECD CECs after exposure to 0.5 mM tBHP (P=0.031 and 0.022, respectively), leading to a 21% increase in TUNEL-positive CECs in FECD (P=0.015) but no change in normal. Baseline p53 expression was twofold higher in FECD than that in normal endothelium (P=0.002). Immunofluorescence revealed an increase in p53 and phospho-p53 levels in FECD compared with that in normal endothelium.
Conclusions: FECD CECs are more susceptible to oxidative DNA damage and oxidative-stress-induced apoptosis than normal. Increased activation of p53 in FECD suggests that it mediates cell death in susceptible CECs. The authors conclude that p53 plays a critical role in complex mechanisms regulating oxidative-stress-induced apoptosis in FECD.
Figures
Figure 1.
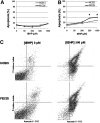
Normal and FECD immortalized cell lines (HCECi and FECDi, respectively) were treated with increasing concentrations of tBHP and stained with Annexin V (Ann) and PI. Early apoptosis (Ann+/PI−) was measured using quantitative flow cytometry. The 4-hour treatment (A) resulted in similar levels of apoptotic cells in both HCECi and FECDi. However, the 14-hour treatment (B) resulted in significantly higher apoptosis in FECDi (*P < 0.05). (C) A representative image of flow cytometric analysis of HCECi and FECDi treated with 500 μM tBHP for 14 hours is illustrated.
Figure 2.
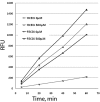
Hydrogen peroxide release from HCECi and FECDi measured using Amplex Red to detect resorufin fluorescence in the cell supernatants. The cells were treated with tBHP, and H2O2 release was detected at different time points as measured by relative fluorescence units (RFUs). All measurements were performed in triplicate. FECDi released a significantly higher level of H2O2 than did HCECi after 20, 40, and 60 minutes of incubation in both untreated (0 μM) and treated (500 μM) conditions. *P < 0.05.
Figure 3.
Normal CECs' resistance to oxidative stress induced by 500 μM tBHP. (A) Densitometric analysis of normal CECs labeled with anti–8-OHdG antibody reveals a significant increase in oxidative DNA damage after 4 hours, but not after 18 hours of tBHP treatment. Despite the increase in oxidative DNA damage, treatment of normal CECs with 500 μM tBHP did not result in significant apoptosis (TUNEL, B), and prolonged (18-hour) treatment with tBHP (C) did not cause significant necrosis (PI), suggesting that CECs are alive. Data are mean ± SEM of tissue from three normal donors per condition (*P = 0.031).
Figure 4.
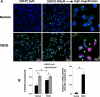
Normal and FECD endothelium treated with tBHP. (A) After treatment, specimens were labeled with TUNEL (red), anti-8-OHdG (green), and TOPRO-3 nuclear stain (blue). Asterisks mark the characteristic guttae of FECD corneal endothelium. Magnifications: ×400 and ×630 with ×6 zoom. (B) Densitometric analysis shows increased levels of 8-OHdG staining in both normal and FECD specimens after treatment, with significantly increased TUNEL-positive staining in FECD (*P < 0.05). Data are mean ± SEM of three normal and three FECD specimens.
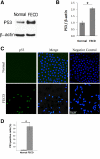
Figure 5.
Increased p53 protein level in FECD compared with normal CECs. (A) A representative Western blot shows increased p53 levels in FECD compared with normal CEC. β-Actin was used for normalization of protein loading. (B) Densitometric analysis of p53 levels in CECs. Data are mean ± SEM of four normal and four FECD specimens (*P = 0.002). (C) Representative confocal images of normal (top row) and FECD (bottom row) endothelium as wholemounts. Nuclear staining of p53 (green) was detected at a higher level in FECD samples compared with normal controls. TOPRO-3 was used for nuclei staining (blue). Overlay of the two channels showed colocalization of p53 and nuclear stain in FECD. Images of negative controls incubated with only secondary antibody are shown in the right column. (D) Densitometric analysis shows increased proportion of p53-positive cells in FECD compared with normal controls (*P < 0.001). Data are mean ± SEM of three normal and three FECD specimens. Original magnification, ×400 with ×2 zoom.
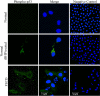
Figure 6.
Representative confocal images of corneal endothelial whole mounts from normal (top row), normal treated with tBHP (middle row), and FECD (bottom row) specimens. Cytoplasmic localization of phospho-p53 (green) was present in normal endothelium after treatment with tBHP and in FECD specimens. TOPRO-3 was used for nuclei staining (blue). Images of negative controls incubated with only secondary antibody are shown in the right column. Original magnification was ×400 with ×6 zoom and ×2 zoom in the first two rows and ×5 zoom and ×3 zoom in the third row.
Similar articles
- Decline in DJ-1 and decreased nuclear translocation of Nrf2 in Fuchs endothelial corneal dystrophy.
Bitar MS, Liu C, Ziaei A, Chen Y, Schmedt T, Jurkunas UV. Bitar MS, et al. Invest Ophthalmol Vis Sci. 2012 Aug 24;53(9):5806-13. doi: 10.1167/iovs.12-10119. Invest Ophthalmol Vis Sci. 2012. PMID: 22836768 Free PMC article. - Sulforaphane decreases endothelial cell apoptosis in fuchs endothelial corneal dystrophy: a novel treatment.
Ziaei A, Schmedt T, Chen Y, Jurkunas UV. Ziaei A, et al. Invest Ophthalmol Vis Sci. 2013 Oct 15;54(10):6724-34. doi: 10.1167/iovs.13-12699. Invest Ophthalmol Vis Sci. 2013. PMID: 24030461 Free PMC article. - Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy.
Jurkunas UV, Bitar MS, Funaki T, Azizi B. Jurkunas UV, et al. Am J Pathol. 2010 Nov;177(5):2278-89. doi: 10.2353/ajpath.2010.100279. Epub 2010 Sep 16. Am J Pathol. 2010. PMID: 20847286 Free PMC article. - Transcript profile of cellular senescence-related genes in Fuchs endothelial corneal dystrophy.
Matthaei M, Zhu AY, Kallay L, Eberhart CG, Cursiefen C, Jun AS. Matthaei M, et al. Exp Eye Res. 2014 Dec;129:13-7. doi: 10.1016/j.exer.2014.10.011. Epub 2014 Oct 11. Exp Eye Res. 2014. PMID: 25311168 Free PMC article. Review. - Mitochondrial Dysfunction and Mitophagy in Fuchs Endothelial Corneal Dystrophy.
Kumar V, Jurkunas UV. Kumar V, et al. Cells. 2021 Jul 26;10(8):1888. doi: 10.3390/cells10081888. Cells. 2021. PMID: 34440658 Free PMC article. Review.
Cited by
- Decline in DJ-1 and decreased nuclear translocation of Nrf2 in Fuchs endothelial corneal dystrophy.
Bitar MS, Liu C, Ziaei A, Chen Y, Schmedt T, Jurkunas UV. Bitar MS, et al. Invest Ophthalmol Vis Sci. 2012 Aug 24;53(9):5806-13. doi: 10.1167/iovs.12-10119. Invest Ophthalmol Vis Sci. 2012. PMID: 22836768 Free PMC article. - Alive and well? Exploring disease by studying lifespan.
Brett JO, Rando TA. Brett JO, et al. Curr Opin Genet Dev. 2014 Jun;26:33-40. doi: 10.1016/j.gde.2014.05.004. Epub 2014 Jul 6. Curr Opin Genet Dev. 2014. PMID: 25005743 Free PMC article. Review. - Nrf2, a Potential Therapeutic Target against Oxidative Stress in Corneal Diseases.
Liu XF, Zhou DD, Xie T, Malik TH, Lu CB, Li HJ, Wang F, Shu C, Liu C, Lu CW, Hao JL. Liu XF, et al. Oxid Med Cell Longev. 2017;2017:2326178. doi: 10.1155/2017/2326178. Epub 2017 Oct 25. Oxid Med Cell Longev. 2017. PMID: 29209447 Free PMC article. Review. - The Interplay Between Metabolites and MicroRNAs in Aqueous Humor to Coordinate Corneal Endothelium Integrity.
Ueno M, Yoshii K, Yamashita T, Sonomura K, Asada K, Ito E, Fujita T, Sotozono C, Kinoshita S, Hamuro J. Ueno M, et al. Ophthalmol Sci. 2023 Mar 16;3(3):100299. doi: 10.1016/j.xops.2023.100299. eCollection 2023 Sep. Ophthalmol Sci. 2023. PMID: 37125267 Free PMC article. - Association of smoking and other risk factors with Fuchs' endothelial corneal dystrophy severity and corneal thickness.
Zhang X, Igo RP Jr, Fondran J, Mootha VV, Oliva M, Hammersmith K, Sugar A, Lass JH, Iyengar SK; Fuchs' Genetics Multi-Center Study Group. Zhang X, et al. Invest Ophthalmol Vis Sci. 2013 Aug 27;54(8):5829-35. doi: 10.1167/iovs.13-11918. Invest Ophthalmol Vis Sci. 2013. PMID: 23882692 Free PMC article.
References
- Borderie VM, Baudrimont M, Vallee A, Ereau TL, Gray F, Laroche L. Corneal endothelial cell apoptosis in patients with Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2000;41:2501–2505 - PubMed
- Li QJ, Ashraf MF, Shen DF, et al. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001;119:1597–1604 - PubMed
- Joyce NC, Zhu CC. Human corneal endothelial cell proliferation: potential for use in regenerative medicine. Cornea. 2004;23:S8–S19 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous