Epigenetic regulation of motor neuron cell death through DNA methylation - PubMed (original) (raw)
Epigenetic regulation of motor neuron cell death through DNA methylation
Barry A Chestnut et al. J Neurosci. 2011.
Abstract
DNA methylation is an epigenetic mechanism for gene silencing engaged by DNA methyltransferase (Dnmt)-catalyzed methyl group transfer to cytosine residues in gene-regulatory regions. It is unknown whether aberrant DNA methylation can cause neurodegeneration. We tested the hypothesis that Dnmts can mediate neuronal cell death. Enforced expression of Dnmt3a induced degeneration of cultured NSC34 cells. During apoptosis of NSC34 cells induced by camptothecin, levels of Dnmt1 and Dnmt3a increased fivefold and twofold, respectively, and 5-methylcytosine accumulated in nuclei. Truncation mutation of the Dnmt3a catalytic domain and Dnmt3a RNAi blocked apoptosis of cultured neurons. Inhibition of Dnmt catalytic activity with RG108 and procainamide protected cultured neurons from excessive DNA methylation and apoptosis. In vivo, Dnmt1 and Dnmt3a are expressed differentially during mouse brain and spinal cord maturation and in adulthood when Dnmt3a is abundant in synapses and mitochondria. Dnmt1 and Dnmt3a are expressed in motor neurons of adult mouse spinal cord, and, during their apoptosis induced by sciatic nerve avulsion, nuclear and cytoplasmic 5-methylcytosine immunoreactivity, Dnmt3a protein levels and Dnmt enzyme activity increased preapoptotically. Inhibition of Dnmts with RG108 blocked completely the increase in 5-methycytosine and the apoptosis of motor neurons in mice. In human amyotrophic lateral sclerosis (ALS), motor neurons showed changes in Dnmt1, Dnmt3a, and 5-methylcytosine similar to experimental models. Thus, motor neurons can engage epigenetic mechanisms to drive apoptosis, involving Dnmt upregulation and increased DNA methylation. These cellular mechanisms could be relevant to human ALS pathobiology and disease treatment.
Figures
Figure 1.
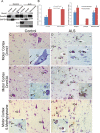
Dnmt protein expression and enzyme activity in non-neural and neural cell lines. A, Immunoblot showing endogenous expression of Dnmt1 and Dnmt3a in cultured HEK293 cells and mouse spinal cord neuron cells (NSC34), mouse brain astrocytes, and mouse brain microglia. B, Negative control antibody preadsorption assay. NSC34 cells were harvested and lysed, and the protein lysates were used for the preadsorption test or nonpreadsorbed controls. Antibody to Dnmt1 or Dnmt3a diluted to 1:1000 (w/v) was incubated with Dnmt1 or Dnmt3a recombinant protein (1:10 and 1:100, ratios of antibody to recombinant protein). After incubation to form the antibody/protein complex, the samples were centrifuged, and the supernatant was collected and used to probe Western blot (20 μg of protein). Ponceau S staining was used to verify protein loading in each lane. C, Cultured HEK and mouse neural cells were transfected with Dnmt1 or Dnmt3a vectors to enforce expression or were mock transfected as a control. The transfected cells were harvested and lysed, and proteins were used for Western blotting. Ponceau S-stained membrane shows protein loading. D, E, RNAi knockdown of Dnmts. NSC34 cells were transiently transfected using siRNAs targeting independently Dnmt1 and Dnmt3a. The cultured cells were harvested 48 h later. siRNA-Dnmt cell lysates (20 μg of total protein) were used for immunoblotting for Dnmt1 and Dnmt3a. The graph displays the Dnmt1 and Dnmt3a immunoreactivity levels as percentage of control optical density (OD; mean ± SD) after siRNA-Dnmt1 and siRNA-Dnmt3a in NSC34 cells (asterisks indicate significant difference from control; see Results). Independent experiments were done in triplicate. F, Knockdown of Dnmt enzyme activity with RNAi. NSC34 whole-cell lysates after Dnmt1- and Dnmt3a-siRNA construct transfection were used to determine Dnmt enzyme activity biochemically. The total enzyme activities are represented as percentage of activity (mean ± SD) compared with control mock-transfected NSC34 cells (asterisks indicate significant difference, p < 0.01). Independent experiments were done twice.
Figure 2.
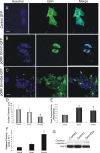
Transient transfection of NSC34 cells with Dnmt-GFP fusion protein expression constructs and induction of neurodegeneration. Cells were transfected, and 48 h later fixed and stained with the nuclear dye Hoechst 33258 (blue). A, Control transfection with GFP expression plasmid (2 μg) shows diffuse green fluorescence throughout NSC34 cells. Scale bar: A, B, 5 μm. B, Cells transfected with plasmid pORF9 Dnmt1-GFP (2 μg) show that Dnmt1 is localized selectively in the nucleus with a striking punctate labeling pattern. C, Cells transfected with plasmid pORF9 Dnmt3a-GFP (2 μg) exhibit nuclear and marked punctate cytoplasmic localizations (open arrows) of Dnmt3a and also show nuclear condensation and fragmentation (hatched arrows). Scale bar, 7 μm. D, Graph showing measurements of nuclear diameters (mean ± SD; asterisk indicates significant difference, p < 0.05) in transfected cells (n = 20 cells per group). E, Total Dnmt enzyme activity (mean ± SD of absorbance) in NSC34 cell lysates after Dnmt1 and Dnmt3a transfection. Asterisks indicate significant difference (p < 0.05) compared with control. F, Caspase-3 enzyme activity in NSC34 cells overexpressing Dmnt1 and Dnmt3a at 24 h after plasmid or mock transfection. Asterisk indicates significant difference (p < 0.05) compared with control. G, Western blot determination of cleaved capsase-3 formation in NSC34 cells overexpressing Dmnt1 and Dnmt3a at 24 h after plasmid or mock transfection. MAP2 immunoreactivity was used as a protein loading control.
Figure 3.
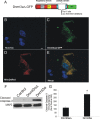
Mutant Dnmt3a-GFP localizes to mitochondria. A, Schematic diagram showing design of mutated Dnmt3a gene construct with a deleted catalytic domain. B–E, Cultured NSC34 cells were cotransfected with Dnmt3aΔ-GFP (C) and mitochondrial-targeted DsRed (D) plasmids and cultured for 48 h to 16 d. Fixed NSC-34 cells were stained with Hoechst 33258 (B). Dnmt3aΔ-GFP was localized to the nucleus and cytoplasm (C). E, Cytoplasmic Dnmt3aΔ-GFP showed near-complete localization to mitochondria (yellow). Scale bar (in B), 7 μm. F, NSC34 cells transfected with catalytic domain mutant Dnmt3a did not show accumulation of cleaved caspase-3 by Western blotting as did cells transfected with wild-type Dnmt3a. MAP2 immunoreactivity was used as a protein loading control. G, Western blot quantification of cleaved caspase-3 immunoreactivity in lysates of NSC34 cells transfected with catalytic domain mutant Dnmt3a (Dnmt3aΔ) and wild-type Dnmt3a compared with mock-transfected control cells. Values (percentage of control) are mean ± SD of three experiments. Asterisk indicates significant difference (p < 0.01) compared with control and Dnmt3aΔ cells.
Figure 4.
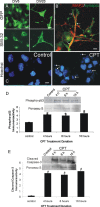
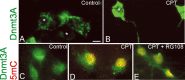
NSC34 neuron morphological characterization and camptothecin-induced apoptosis. A, Morphological characterization of the differentiation of cultured NSC34 cells. NSC34 cells were cultured for 5 d (DIV5) and differentiated over 20 d (DIV20). Living cells at DIV5 and DIV20 were transfected with GFP expression plasmid or were fixed and used for immunofluorescence for neurofilament protein with SMI-32 antibody. Immature DIV5 NSC34 cells are small and bipolar as seen by GFP expression and neurofilament staining. In contrast, differentiated DIV20 NSC34 cells have features of motor neurons with large multipolar cell bodies and broad dendrites. Over a 20 d culture, the original plating density is reduced ∼50%. Scale bar, 10 μm. B, Differentiated NSC34 neurons form elaborate dendrites (red, MAP2) contacted by numerous synapses (hatched arrows, green, synaptophysin). Scale bar, 5 μ
m
. C–E, DIV20 NSC34 cells treated with 10 μ
m
CPT, a DNA topoisomerase I inhibitor that induces DNA single-strand breaks (Martin et al., 2009b), undergo robust apoptosis. C, Hoechst 33258 staining shows that nuclei of NSC34 cells treated with CPT undergo apoptosis and show nuclear condensation, shrinkage, and fragmentation (hatched arrows). The same batch of DIV20 cultures treated with vehicle (control) has normal large nuclei. Scale bar, 10 μm. D, E, Whole-cell lysates of DIV20 NSC34 cells treated with 10 μ
m
CPT show increased immunoreactivity for activated phospho-p53 (D) and cleaved-caspase 3 (E) at 4, 8, and 16 h of CPT treatment compared with vehicle-treated control cells. Values in graphs are mean ± SEM of optical density units. Asterisks denote a significant difference from control for phospho-53 (p < 0.01) and cleaved caspase-3 (p < 0.001). Representative Western blots are shown along with Ponceau S-stained membranes to show protein loading. The results were replicated in at least three separate cell culture experiments.
Figure 5.
Dnmts can mediate DNA damage-induced apoptosis of differentiated neurons. A, B, Dnmt1 and Dnmt3a protein levels do not change significantly in DIV5 NSC34 cells exposed to 10 μ
m
CPT for 4–24 h. Representative Western blots are shown along with membranes reprobed for actin to show protein loading. Immunoreactivity optical densities for DNMT1 and Dnmt3a were normalized to actin immunoreactivity. Values in graphs are mean ± SEM of optical density units. C, D, Dnmt1 and Dnmt3a protein levels increase in DIV20 NSC34 cells exposed to 10 μ
m
CPT for 4–24 h. Values in graphs are mean ± SEM of optical density units. Asterisks denote a significant difference from control for Dnmt1 (p < 0.01) and Dnmt3a (p < 0.05). Representative Western blots are shown along with membranes reprobed for actin to show protein loading. E, Dnmt inhibitors protect against CPT-induced apoptosis. DIV20 NSC34 cells were treated with either 20 μ
m
RG108 or 0.5 m
m
procainamide for 2.5 h in optimem medium. The medium with inhibitors was removed, and the cells were treated with 10 μ
m
CPT for 24 h. The cells were fixed and stained with Hoechst 33258. The percentages of apoptotic cells were determined by counting total cell nuclei (n = 1000 cells per condition) and the proportion of apoptotic nuclei. Values in graphs are mean ± SEM. Double asterisks denote a significant difference from control for CPT (p < 0.001). There is a significant difference from CPT-treated cells for RG108 (++p < 0.01) and procainamide (+p < 0.05). F, RNAi for Dnmt3a protects against CPT-induced apoptosis. DIV14 NSC34 cells were transfected with siRNA construct targeting Dnmt3a or BACE1 (as an independent target), and 24 h later, the cells were treated with 10 μ
m
CPT for 24 h. Cells were prepared as in E. Values in graphs are mean ± SEM. ++Significant difference from control for CPT (p < 0.001); +Significant difference from CPT-treated cells for siRNAi cells (p < 0.01). BACE1 knockdown (control siRNA + CPT) did not protect. G, Western blot analysis of phospho-p53Ser15 in NSC34 cells treated with RG108 (20 μ
m
) and Dnmt3a-siRNA and challenged with 10 μ
m
CPT for 24 h. RG108 dramatically blocked the CPT-induced activation of p53. Dnmt3a-siRNA attenuated the activation of p53 in CPT-treated cells.
Figure 6.
Cellular localization of endogenous Dnmt3a and 5-methylcytosine in NSC34 cells treated with CPT. A, Immunofluorescence for Dnmt3a in control DIV20 NSC34 cells shows its localization mostly in the cytoplasm, some of which are seen as discrete puncta, with low detection in the nucleus (asterisks). B, In DIV20 NSC34 cells treated with 10 μ
m
CPT, Dnmt3a immunoreactivity is highly enriched in the cytoplasm and processes and is also present robustly in the nucleus (asterisk). C–E, Immunofluorescent colocalization of Dnmt3a (green) and 5-mC (red) in control (C) and CPT-treated DIV14 NSC34 (D, E) cells. C, Control cells have a primarily cytoplasmic perinuclear localization of Dnmt3a (green) and very low 5-mC (red) limited to a few faint specks in the nuclei, some of which are also faintly yellow because of colocalization with 5-mC. D, In contrast, CPT-treated cells have very high levels of immunoreactivity for both Dnmt3 and 5-mC as witnessed by the intense yellow indicating colocalization. E, RG108 treatment attenuated markedly the immunoreactivities of both Dnmt3a and 5-mC in CPT-treated cells as indicated by the dissipation of the yellow signal. Scale bar (in A), 6 μm.
Figure 7.
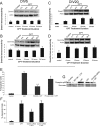
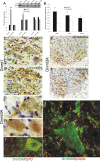
Developmental expression, regional distribution, and subcellular localization of Dnmt1 and Dnmt3a in mouse CNS. A, Dnmt1 protein levels are very stable during normal postnatal development of mouse brain, whereas Dnmt3a levels increase. Mouse whole brains at P0 (day of birth) through P30 and into adulthood (8 weeks) were homogenized and used for immunoblotting for Dnmt1 and Dnmt3a. Membranes were stained with Ponceau S to show protein loading. Values in graphs are mean ± SEM of optical density units. Asterisks denote a significant difference from P0 for Dnmt3a (p < 0.05). B, Dnmt1 protein levels decrease during postnatal development of mouse spinal cord, whereas Dnmt3a levels increase. Mouse whole spinal cords at P0 (day of birth) through P20 were homogenized and used for immunoblotting for Dnmt1 and Dnmt3a. Membranes were stained with Ponceau S to show protein loading. Values in graphs are mean ± SEM of optical density units. Asterisks denote a significant reduction (p < 0.05) from P0 for Dnmt1 and a significant increase (p < 0.05) from P0 for Dnmt3a. C, Dnmt1 protein levels are uniform within different regions of adult mouse brain, whereas Dnmt3a levels show regional differences. Adult mouse brains were carefully microdissected into different regions that were homogenized and used for immunoblotting for Dnmt1 and Dnmt3a. Membranes were stained with Ponceau S to show protein loading. Values in graphs are mean ± SEM of optical density units. D, Dnmt1 is found primarily in the nucleus and cytosol, whereas Dnmt3a is found in the nucleus, cytosol, and mitochondria. Adult mouse whole brains and whole spinal cords were homogenized; fractionated into nuclear (Nuc), cytosol (Cyto), and pure mitochondria (Mito) compartments; and used for immunoblotting for Dnmt1 and Dnmt3a. In both brain and spinal cord, the nuclear enrichment was confirmed by NeuN immunoreactivity, and mitochondrial enrichment was confirmed by mitochondrial complex V immunoreactivity. Ponceau S-stained nitrocellulose membranes show protein loading. Values in graphs are mean ± SEM of optical density units.
Figure 8.
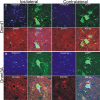
Dnmts are upregulated in adult mouse spinal cord during motor neuron apoptosis. A, Dnmt3a protein levels in spinal cord increase ipsilaterally after SNA. Adult mice received unilateral SNA, and 3, 4, and 5 d later (n = 4 per time), the lumbar spinal cords were microdissected into the nonlesioned contralateral control ventral horn (contra) and the lesioned ipsilateral ventral horn (ipsi). Crude homogenates were used for immunoblotting for Dnmt1 and Dnmt3a. Membranes were stained with Ponceau S to show protein loading. Values in graphs are mean ± SEM of optical density units. Asterisks denote a significant difference from contralateral for Dnmt3a at 3 d (p < 0.001) and 4 d (p < 0.05). See Figure 7_B_ for basal levels of Dnmt1 and Dnmt3a in mature (P20) whole mouse spinal cord. B, Dnmt enzyme activity in spinal cord increases ipsilaterally after SNA. Adult mice received unilateral SNA, and 3, 4, and 5 d later (n = 4 per time), the lumbar spinal cords were microdissected into the nonlesioned contralateral control ventral horns (contra) and the lesioned ipsilateral ventral horns (ipsi). Crude homogenates were used for biochemical assessment of total Dnmt enzyme activity. Values in graphs are mean ± SD. Asterisks denote a significant difference from contralateral at 3 and 4 d (p < 0.01) and at 5 d (p < 0.05). C, D, Immunohistochemical localization of Dnmt1 in mouse lumbar spinal cord at 4 d after SNA. Dnmt1 immunoreactivity was visualized with diaminobenzedine (brown), and sections were counterstained with cresyl violet. C, The ipsilateral ventral horn showed Dnmt1 immunoreactivity within the cytoplasm and nucleus of motor neurons (arrow and inset) and in small cells resembling microglia (hatched arrows). Some motor neurons with chromatin condensation typical of apoptosis (as seen by cresyl violet counterstaining) displayed aggregates of Dnmt1 immunoreactivity in the nucleus and perinuclear region (inset; black circle delineates the nucleus). Scale bars: C (same for D), 20 μm; C, inset, 10 μm. D, In contrast, the contralateral ventral horn showed Dnmt1 immunoreactivity diffusely in the neuropil. E–H, Immunohistochemical localization of Dnmt3a in mouse lumbar spinal cord at 3 d after SNA as seen at low (E, F) and high (G, H) magnifications. Dnmt3a immunoreactivity was visualized with diaminobenzedine (brown), and sections were counterstained with cresyl violet. E, The contralateral control ventral horn showed Dnmt3a immunoreactivity within and around motor neurons (open arrows), but the nuclei of most motor neurons have low immunoreactivity in the nucleus. G, As seen at high resolution, Dnmt3a in normal motor neurons was seen faintly as diffuse immunoreactivity in the nucleus (asterisk) and in the cytoplasm as discrete puncta (hatched arrows). Pericellular bouton-like Dnmt3a immunoreactivity appeared in apposition to motor neurons (open arrows). F, H, In contrast, most ipsilateral motor neurons (F, open arrows) showed prominent Dnmt3a immunoreactivity in the nucleus (H, asterisks). Scale bars: in E (same for F), 20 μm; in G (same for H, I), 5 μm I, Confocal microscopy shows that some cytoplasmic Dnmt3a immunoreactivity in normal motor neurons is localized to mitochondria as demonstrated by its colocalization with the mitochondrial marker cyclophilin D (CyPD). Dnmt3a immunoreactivity is seen as green, and CyPD immunoreactivity is seen as red. Some cytoplasmic Dnmt3a immunoreactivity associates with mitochondria (seen as yellow), but single signals for Dnmt3a and CyPD can also be seen. Dnmt3a immunoreactivity is also seen in the nucleus (asterisk). J, Confocal microscopy shows that some Dnmt3a immunoreactivity is localized to presynaptic terminals as demonstrated by its colocalization with the presynaptic terminal marker synaptophysin. Dnmt3a immunoreactivity is seen as green, and synpatophysin (Synapto) immunoreactivity is seen as red. Perineuronal and peridendritic Dnmt3a is localized to axon terminals (hatched arrows, seen a yellow). Scale bar, 10 μm.
Figure 9.
SNA lesions in transgenic mice expressing eGFP selectively in motor neurons. Hb9-eGFP transgenic mice confirm the expression of Dnmt1 (A) and Dnmt3a (B) in adult motor neurons and their upregulation in ipsilateral motor neurons after SNA compared with contralateral nonlesioned motor neurons, and they potentially serve as a reported model for DNA methylation silencing of gene expression during apoptosis. Motor neurons are labeled genetically with eGFP. Dnmt1 or Dnmt3a immunoreactivities are seen as red, and nuclear labeling is blue (Hoechst). Some ipsilateral lumbar motor neurons can be found with a high eGFP signal (A, B, open arrows), whereas in other motor neurons, the eGFP signal is attenuated or nearly abolished (A, B, hatched arrows). Motor neurons with greater Dnmt1 or Dnmt3a signal generally have a lower eGFP signal (hatched arrows). Dnmt1 immunoreactivity was also seen associated with small cells (A, double arrow). Dnmt3a immunoreactivity was also upregulated in dendrites of motor neurons (B, double arrow). Scale bars, 7 μm.
Figure 10.
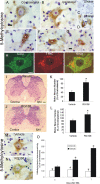
DNA methylation increases in adult mouse motor neurons undergoing apoptosis, and Dnmt inhibition blocks the DNA methylation and the apoptosis. A, B, Immunohistochemical localization of 5-mC in nonlesioned contralateral (A) and SNA-lesioned (B) lumbar spinal cord motor neurons. 5-mC immunoreactivity was visualized with diaminobenzedine (brown), and sections were counterstained with cresyl violet. In control motor neurons (A), 5-mC immunoreactivity was present in the cytoplasm as puncta and in the nucleus as puncta and finely beaded strands. In motor neurons at 4 d after SNA (B), 5-mC immunoreactivity was enriched in the cytoplasm and nucleus. Scale bar (same for B), 7 μm. C, DNase digestion of SNA spinal cord sections abolished completely the 5-mC immunoreactivity in motor neurons. Scale bar (same for D), 10 μm. D, D. RNase digestion of SNA spinal cord sections did not alter the 5-mC immunoreactivity in motor neurons. E–G, Adult motor neurons at different morphological stages of the apoptotic process (Martin et al., 1999; Martin and Liu, 2002) show marked changes in 5-mC immunoreactivity. E, In the chromatolytic stage, the nucleus becomes completely positive for 5-mC immunoreactivity (asterisk) and large aggregates of 5-mC accumulate in the cytoplasm. F, In the somatodendritic attrition stage, characterized by cell-body shrinkage and loss of processes, the nucleus remains strongly positive for 5-mC (asterisk), and 5-mC immunoreactive debris is found in the neuropil (arrow). G, At near end stage, characterized by a residual cell body without processes, the 5-mC immunoreactivity dissipates, and the nucleus is seen as fragments of 5-MeC immunoreactivity (asterisk). H, Confocal microscopy shows that some cytoplasmic 5-mC immunoreactivity in nonlesioned motor neurons is localized to mitochondria, as demonstrated by its colocalization with the mitochondrial marker SOD2. 5-mC immunoreactivity is seen as red, and SOD2 immunoreactivity is seen as green. Much of the cytoplasmic Dnmt3a immunoreactivity associates with mitochondria (seen as yellow), but single signals for 5-mC and SOD2 can also be seen. Scale bar, 10 μm. I, J, Dnmt inhibitor RG108 completely blocks the degeneration of adult motor neurons after SNA. Mice were treated intracerebroventricularly with 10 μg of RG108 (in 2 μl of sterile saline) or with saline (vehicle) on day 0 (day of lesion) and on every other postlesion day over 3 weeks and were killed at 21 d after SNA. I, Vehicle-treated mice show loss of motor neurons in the ventral horn ipsilateral to the lesion. J, RG108-treated mice show no loss of motor neurons after SNA. Circles delineate lateral ventral horn regions. Scale bar, 120 μm. K, Graph showing motor neuron cell counts in SNA mice treated with vehicle (saline) or RG108 and that survived for 21 d. Values (mean ± SD) are represented as percentage of the ipsilateral motor neuron number relative to the contralateral nonlesioned side. The asterisk denotes significant difference (p < 0.01) compared with vehicle. L, Graph showing motor neuron cell volumes in SNA mice treated with vehicle (saline) or RG108 and that survived for 21 d. Values (mean ± SD) are represented as percentage of the remaining ipsilateral motor neuron volumes relative to the motor neuron volumes in the contralateral nonlesioned side. The asterisk denotes significant difference (p < 0.01) compared with vehicle. M, N, Dnmt inhibitor RG108 completely blocks the rise in DNA methylation in adult motor neurons after SNA. Mice were treated intracerebroventricularly with 10 μg of RG108 (in 2 μl of sterile saline) or with saline (vehicle) on day 0 (day of lesion) and days 2 and 4 and were killed at 4 d after SNA. Ipsilateral lumbar motor neurons in vehicle mice displayed an increase in 5-MeC immunoreactivity in the nucleus and cytoplasm (M), and this rise in 5-mC immunoreactivity was blocked by RG108 (N). Scale bar, 7 μm. O, Graph showing motor neuron 5-mC immunoreactivity in SNA mice treated with vehicle (saline) or RG108 and that survived for 3, 4 or 7 d. Quantification was done by single-cell densitometry (Martin et al., 2005). Values (mean ± SD) are the levels of 5-mC immunoreactivity in ipsilateral motor neurons as a percentage of the levels of 5-mC immunoreactivity in contralateral motor neurons. Asterisks denote significant differences at 3, 4, and 7 d (p < 0.05, p < 0.01, and p < 0.001, respectively) compared with vehicle. Plus signs denotes significant differences at 4 and 7 d (p < 0.01, and p < 0.001, respectively) compared with 3 d vehicle.
Figure 11.
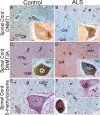
Dnmts and 5-mC are upregulated in human sporadic ALS motor cortex. A, Westerns blots for Dnmt1 and Dnmt3a in human control and sporadic ALS primary motor cortex homogenates fractionated into nuclear-enriched, soluble, and mitochondrial-enriched protein compartments. The blots were reprobed for GAPDH to show protein loading. B, Graphs showing the analysis of Dnmt1 and Dnmt3a Western blots on human control and ALS primary motor cortex tissue fractions. Values represent mean ± SD of optical density (OD) units. Dnmt1 was barely detectable or undetectable in control mitochondrial fractions and was undetectable in control and ALS soluble fractions. Dnmt3a was barely detectable or undetectable in control soluble fractions. Asterisks denote significant differences (p < 0.05) compared with control. C, D, Immunohistochemical localization of 5-mC in human control (C) and ALS (D) motor cortex. Panoramic images of deep layers and higher-magnification images (insets) are shown. C, In control motor cortex, Dnmt1 immunoreactivity is very low to not detectable in neurons, including large Betz cells (open arrows), but is present in small non-neuronal cells (inset, hatched arrows). D, In ALS motor cortex, numerous neurons are distinctly positive for Dnmt1 (open arrows). Dnmt1 immunoreactivity is enriched in the nucleus of some neurons (inset, open arrows and asterisk), but nearby neurons have scant immunoreactivity (inset hatched arrow). Scale bars: (in C, same for D–H), 22 μm; (in A, inset; same for D–H, insets), 10 μm. E, F, Immunohistochemical localization of Dnmt3a in human control (E) and ALS (F) motor cortex. E, In control motor cortex, Dnmt3a immunoreactivity is present in the cytoplasm of neurons (open arrows), including Betz cells, and is present in non-neuronal cells (inset, hatched arrow) and in the neuropil as fine puncta. The nucleus of control neurons has low Dnmt3a immunoreactivity (inset asterisk). F, In ALS motor cortex, numerous neurons, including Betz cells, are distinctly positive for Dnmt3a (open arrows). Dnmt3a immunoreactivity is enriched in the nucleus of some neurons (inset, open arrow and asterisk), but nearby cells are negative (inset, hatched arrow). G, H, Immunohistochemical localization of 5-mC in human control (G) and ALS (H) motor cortex. 5-mC immunoreactivity was visualized with diaminobenzedine (brown), and sections were counterstained with cresyl violet. G, In control motor cortex, most pyramidal neurons (open arrows) have low 5-mC immunoreactivity. 5-mC immunoreactivity was present faintly in pyramidal neurons (inset, open arrow) as fine particles in the nucleus (asterisk) and cytoplasm, but in nearby glial cells (inset, hatched arrows), 5-mC immunoreactivity was enriched in the nucleus. H, In ALS motor cortex, many pyramidal neurons (open arrows) show obvious 5-mC immunoreactivity. 5-mC immunoreactivity was enriched in the nucleus (inset, asterisk) of pyramidal neurons (open arrow), whereas nearby glial cells (inset, hatched arrows) showed scant 5-mC immunoreactivity.
Figure 12.
Localizations of Dnmts and 5-mC in human control and sporadic ALS spinal motor neurons. A, B, Immunohistochemical localization of Dnmt1 in human control (A) and ALS (B) spinal motor neurons. Dnmt1 immunoreactivity was visualized with diaminobenzedine (brown), and sections were counterstained with cresyl violet. Panoramic images of several motor neurons in ventral horn and single-cell high-magnification images (insets) are shown. A, In control ventral horn, Dnmt1 immunoreactivity localizes to motor neurons (open arrows), small non-neuronal cells, and the neuropil. In control motor neurons (inset) Dnmt1 immunoreactivity is found in the nucleus (asterisk) as clusters around the nuclear envelop and as fine strands. In the cytoplasm, Dnmt1 immunoreactivity is seen conspicuously as large aggregates peripherally located (open arrow). B, In ALS ventral horn, Dnmt1 immunoreactivity is conspicuous in attritional motor neurons (open arrows). In ALS motor neurons (inset) Dnmt1 is enriched completely throughout the nucleus (asterisk) and in the cytoplasm where it can be seen as puncta (hatched arrows). Scale bars, (in A, same for B–F), 7 μm; (in A, inset; same for B–F, insets), 7 μm. C, D, Immunohistochemical localization of Dnmt3a in human control (C) and ALS (D) spinal motor neurons. Dnmt3a immunoreactivity was visualized with diaminobenzedine (brown) and sections were counterstained with cresyl violet. C, In control ventral horn, Dnmt3a immunoreactivity localizes to motor neurons (open arrows) and small non-neuronal cells. In control motor neurons (inset), Dnmt3a immunoreactivity is present in the cytoplasm, where it is seen as numerous mitochondria-like structures, and is less enriched in the nucleus (asterisk), where it associates with the nucleolus (hatched arrow) and strand-like structures (double arrow). Dnmt3a is also present throughout the neuropil in a bouton-like pattern (open arrows). D, In ALS ventral horn, Dnmt3a immunoreactivity is highly enriched in attritional motor neurons (open arrows) and in small non-neuronal cells. In ALS motor neurons (inset), Dnmt3a immunoreactivity is enriched in the perinuclear cytoplasm and also in the nucleus (asterisk), where it is aggregated around the nucleolus (hatched arrow) and throughout the nuclear matrix (double arrow). E, F, Immunohistochemical localization of 5-mC in human control (E) and ALS (F) spinal motor neurons. 5-mC immunoreactivity was visualized with diaminobenzedine (brown), and sections were counterstained with cresyl violet. E, In control ventral horn, 5-mC immunoreactivity is present in motor neurons (open arrows) and in numerous small non-neuronal cells. In control motor neurons (inset) 5-mC immunoreactivity is found in the nucleus (asterisk) as small aggregates and strands near the nuclear envelope (double arrow) and nucleolus. In the cytoplasm of motor neurons, 5-mC immunoreactivity is seen as round particles. F, In ALS ventral horn, 5-mC immunoreactivity is conspicuous in attritional motor neurons (open arrows). In ALS residual motor neurons, 5-mC is enriched throughout the nucleus in some cells (top right inset, asterisk) and in other cells, 5-mC completely fills the nucleus and cytoplasm (bottom left inset).
Similar articles
- Aberrant regulation of DNA methylation in amyotrophic lateral sclerosis: a new target of disease mechanisms.
Martin LJ, Wong M. Martin LJ, et al. Neurotherapeutics. 2013 Oct;10(4):722-33. doi: 10.1007/s13311-013-0205-6. Neurotherapeutics. 2013. PMID: 23900692 Free PMC article. Review. - Aberrant DNA and RNA Methylation Occur in Spinal Cord and Skeletal Muscle of Human SOD1 Mouse Models of ALS and in Human ALS: Targeting DNA Methylation Is Therapeutic.
Martin LJ, Adams DA, Niedzwiecki MV, Wong M. Martin LJ, et al. Cells. 2022 Oct 31;11(21):3448. doi: 10.3390/cells11213448. Cells. 2022. PMID: 36359844 Free PMC article. - Mitochondrial DNMT3A and DNA methylation in skeletal muscle and CNS of transgenic mouse models of ALS.
Wong M, Gertz B, Chestnut BA, Martin LJ. Wong M, et al. Front Cell Neurosci. 2013 Dec 25;7:279. doi: 10.3389/fncel.2013.00279. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24399935 Free PMC article. - Effects of DNMT1 and HDAC inhibitors on gene-specific methylation reprogramming during porcine somatic cell nuclear transfer.
Xu W, Li Z, Yu B, He X, Shi J, Zhou R, Liu D, Wu Z. Xu W, et al. PLoS One. 2013 May 31;8(5):e64705. doi: 10.1371/journal.pone.0064705. Print 2013. PLoS One. 2013. PMID: 23741375 Free PMC article. - DNA methyltransferases in hematological malignancies.
Hoang NM, Rui L. Hoang NM, et al. J Genet Genomics. 2020 Jul 20;47(7):361-372. doi: 10.1016/j.jgg.2020.04.006. Epub 2020 Jul 24. J Genet Genomics. 2020. PMID: 32994141 Free PMC article. Review.
Cited by
- Matters of life and death: the role of chromatin remodeling proteins in retinal neuron survival.
Lagali PS, Picketts DJ. Lagali PS, et al. J Ocul Biol Dis Infor. 2011 Sep;4(3):111-20. doi: 10.1007/s12177-012-9080-3. Epub 2012 Mar 17. J Ocul Biol Dis Infor. 2011. PMID: 23289056 Free PMC article. - Epigenetic mechanisms in neurological disease.
Jakovcevski M, Akbarian S. Jakovcevski M, et al. Nat Med. 2012 Aug;18(8):1194-204. doi: 10.1038/nm.2828. Nat Med. 2012. PMID: 22869198 Free PMC article. Review. - DNA methyltransferase 1 mutations and mitochondrial pathology: is mtDNA methylated?
Maresca A, Zaffagnini M, Caporali L, Carelli V, Zanna C. Maresca A, et al. Front Genet. 2015 Mar 12;6:90. doi: 10.3389/fgene.2015.00090. eCollection 2015. Front Genet. 2015. PMID: 25815005 Free PMC article. - The Role of Epigenetic Modifications in Neurotoxicity Induced by Neonatal General Anesthesia.
Ma LH, Yan J, Jiao XH, Zhou CH, Wu YQ. Ma LH, et al. Front Mol Neurosci. 2022 Apr 27;15:877263. doi: 10.3389/fnmol.2022.877263. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35571375 Free PMC article. Review. - Lithium reduces the effects of rotenone-induced complex I dysfunction on DNA methylation and hydroxymethylation in rat cortical primary neurons.
Scola G, Kim HK, Young LT, Salvador M, Andreazza AC. Scola G, et al. Psychopharmacology (Berl). 2014 Oct;231(21):4189-98. doi: 10.1007/s00213-014-3565-7. Epub 2014 Apr 29. Psychopharmacology (Berl). 2014. PMID: 24777143
References
- Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–2402. - PubMed
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. - PubMed
- Brenner C, Fuks F. DNA methyltransferases: facts, clues, mysteries. Curr Top Microbiol Immunol. 2006;301:45–66. - PubMed
- Brueckner B, Garcia Boy R, Siedlecki P, Musch T, Kliem HC, Zielenkiewicz P, Suhai S, Wiessler M, Lyko F. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 2005;65:6305–6311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS065895-01/NS/NINDS NIH HHS/United States
- NS034100/NS/NINDS NIH HHS/United States
- R01 NS052098/NS/NINDS NIH HHS/United States
- NS052098/NS/NINDS NIH HHS/United States
- R01 AG016282-01A1/AG/NIA NIH HHS/United States
- R01 NS034100/NS/NINDS NIH HHS/United States
- R01 NS079348/NS/NINDS NIH HHS/United States
- R01 NS052098-02/NS/NINDS NIH HHS/United States
- AG016282/AG/NIA NIH HHS/United States
- R01 AG016282/AG/NIA NIH HHS/United States
- NS065895/NS/NINDS NIH HHS/United States
- R01 NS065895/NS/NINDS NIH HHS/United States
- R01 NS034100-04/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous