Age related changes in microglial phenotype vary between CNS regions: grey versus white matter differences - PubMed (original) (raw)
Age related changes in microglial phenotype vary between CNS regions: grey versus white matter differences
Adam D Hart et al. Brain Behav Immun. 2012 Jul.
Abstract
Subtle regional differences in microglial phenotype exist in the adult mouse brain. We investigated whether these differences were amplified during ageing and following systemic challenge with lipopolysaccharide (LPS). We studied microglial morphology and phenotype in young (4mo) and aged (21mo) C57/BL6 mice using immunohistochemistry and quantified the expression levels of surface molecules on microglia in white and grey matter along the rostral-caudal neuraxis. We detected significant regional, age dependent differences in microglial phenotypes, with the microglia of white matter and caudal areas of the CNS exhibiting greater upregulation of CD11b, CD68, CD11c, F4/80 and FcγRI than grey matter and rostral CNS areas. Upregulation of CD11c with age was restricted to the white matter, as was the appearance of multinucleated giant cells. Systemic LPS caused a subtle upregulation of FcγRI after 24 h, but the other markers examined were not affected. Burrowing behaviour and static rod assays were used to assess hippocampal and cerebellar integrity. Aged mice exhibited exaggerated and prolonged burrowing deficits following systemic LPS injection, while in the absence of an inflammatory challenge aged mice performed significantly worse than young mice in the static rod test. Taken together, these findings show that the effects of age on microglial phenotype and functional integrity vary significantly between CNS compartments, as do, albeit to a lesser extent, the effects of systemic LPS.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Supplementary Fig. 1
Supplementary Fig. 2
Fig. 1
CD11b and CD68 marker expression increases with age and LPS. (A–H) CD11b. (A–D) Hippocampus. A – 4 month old saline, B – 4 month old LPS, C – 21 month old saline, D – 21 month old LPS. (E–G) Cerebellum. E – 4 month old saline, F – 4 month old LPS, G – 21 month old saline, H – 21 month old LPS. (I–P) CD68. (I–L) Hippocampus. I – 4 month old saline, J – 4 month old LPS, K – 21 month old saline, L – 21 month old LPS. (M–P) Cerebellum. M – 4 month old saline, N – 4 month old LPS, O – 21 month old saline, P – 21 month old LPS.
Fig. 2
Microglial membrane marker expression increases with age. (A, B) Double immunofluorescence staining for CD11b (green) and collagen IV (red) in the fimbria (A) and cerebellar inferior peduncle (B) of a 21 month old mouse. CD11b positive cell aggregates are indicated by white arrows. The aggregates contain multiple nuclei and are not situated directly adjacent to blood vessels, as indicated by collagen IV positive basement membranes. Nuclei are blue after DAPI staining. (C) Double immunofluorescence staining for CD11c (green) and Ki67 (red), a marker of proliferation, in the cerebellar inferior peduncle. CD11c positive aggregates do not co-localise with Ki67 positive nuclei. D&G – CD11c staining in the cerebellar inferior peduncle of a 21 month old (D) and 4 month old (G) mouse. E&H – FcγRI staining in the cerebellar inferior peduncle of a 21 month old (E) and 4 month old (H) mouse. F&I – F4/80 staining in the cerebellar inferior peduncle of a 21 month old (F) and 4 month old (I) mouse.
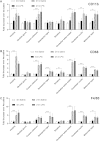
Fig. 3
Age related changes in microglial phenotype depends on brain region. Tissue collected and analysed 24 h after intraperitoneal injection of saline or LPS. (A) CD11b expression in selected regions of the CNS. CD11b expression was significantly influenced by age, region and age × region (p < 0.05), but not LPS. n = 4–5 per region. (B) CD68 expression in selected regions of the CNS. CD68 expression was significantly influenced by age, region and age × region, but not LPS (p < 0.001). n = 4–5 per region. (C) F4/80 expression in selected regions of the CNS. F4/80 expression was significantly influenced by age, region and age × region (p < 0.001), but not LPS. n = 4–5 per region. Horizontal bars denote a significant effect of age (4 m saline to 21 m saline) or LPS (21 m saline to 21 m LPS) within that region. Data is shown as mean ± SEM. ∗ denotes p < 0.05; ∗∗ denotes p < 0.01; ∗∗∗ denotes p < 0.001.
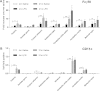
Fig. 4
Age related changes in CD11c and FcγRI expression depends on brain region. Tissue collected and analysed 24 h after intraperitoneal injection of saline or LPS. (A) CD11c expression in selected regions of the CNS. CD11c expression was significantly influenced by age, region and age × region (p < 0.001), but not LPS. n = 5 per region. (B) FcγRI expression in selected regions of the CNS. FcγRI expression was significantly influenced by region, age and LPS (p < 0.05). n = 4–5 mice per region. Horizontal bars denote a significant effect of age (4 m saline to 21 m saline) or LPS (21 m saline to 21 m LPS) within that region. Data is shown as mean ± SEM. ∗ denotes p < 0.05; ∗∗ denotes p < 0.01; ∗∗∗ denotes p < 0.001.
Fig. 5
Behavioural changes in young and aged mice and the effect of systemic inflammation. (A) Burrowing measured between 3 and 5 h after intraperitoneal injection of LPS (100 μg/kg) or saline. Data shows systemic LPS induced a greater decline in 21 month old mice compared to 4 month old mice. Horizontal bars represent medians. (B) Overnight burrowing after intraperitoneal injection of LPS or saline. Data shows that recovery of burrowing is slower in 21 month old mice compared to 4 month old mice. Horizontal bars represent means. (C) Percentage fail rate in the 9 mm static rod test measured at baseline or between 1 and 2 h after intraperitoneal LPS or saline injection. 21 month old mice failed more frequently in the 9 mm static rod test than 4 months old. This change in performance was independent of strength (Supplementary Fig. 2). Pass/fail ratios were not influenced by injection of saline (open bars) or LPS (black, filled bars). Columns represent the percentage of mice which fail the task. As this graph is representation of binomial data drawn from a single experiment, no error bars are presented. (D) 9 mm static rod transit time to reach platform at baseline. Transit times reflect a decline in performance with age. Data is shown as mean ± SEM. ∗∗∗p < 0.001. Letters denote a significant difference between groups (p < 0.05).
Similar articles
- Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide.
Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Corona AW, et al. J Neuroinflammation. 2010 Dec 17;7:93. doi: 10.1186/1742-2094-7-93. J Neuroinflammation. 2010. PMID: 21167054 Free PMC article. - Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease.
Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Cunningham C, et al. Biol Psychiatry. 2009 Feb 15;65(4):304-12. doi: 10.1016/j.biopsych.2008.07.024. Epub 2008 Sep 18. Biol Psychiatry. 2009. PMID: 18801476 Free PMC article. - Age- and location-related changes in microglial function.
Ritzel RM, Patel AR, Pan S, Crapser J, Hammond M, Jellison E, McCullough LD. Ritzel RM, et al. Neurobiol Aging. 2015 Jun;36(6):2153-63. doi: 10.1016/j.neurobiolaging.2015.02.016. Epub 2015 Feb 23. Neurobiol Aging. 2015. PMID: 25816747 - Systemic inflammation and microglial activation: systematic review of animal experiments.
Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Hoogland IC, et al. J Neuroinflammation. 2015 Jun 6;12:114. doi: 10.1186/s12974-015-0332-6. J Neuroinflammation. 2015. PMID: 26048578 Free PMC article. Review. - Region-Specific Phenotypes of Microglia: The Role of Local Regulatory Cues.
De Biase LM, Bonci A. De Biase LM, et al. Neuroscientist. 2019 Aug;25(4):314-333. doi: 10.1177/1073858418800996. Epub 2018 Oct 3. Neuroscientist. 2019. PMID: 30280638 Review.
Cited by
- Reducing inflammation and rescuing FTD-related behavioral deficits in progranulin-deficient mice with α7 nicotinic acetylcholine receptor agonists.
Minami SS, Shen V, Le D, Krabbe G, Asgarov R, Perez-Celajes L, Lee CH, Li J, Donnelly-Roberts D, Gan L. Minami SS, et al. Biochem Pharmacol. 2015 Oct 15;97(4):454-462. doi: 10.1016/j.bcp.2015.07.016. Epub 2015 Jul 20. Biochem Pharmacol. 2015. PMID: 26206194 Free PMC article. - Toward a human brain extracellular vesicle atlas: Characteristics of extracellular vesicles from different brain regions, including small RNA and protein profiles.
Huang Y, Arab T, Russell AE, Mallick ER, Nagaraj R, Gizzie E, Redding-Ochoa J, Troncoso JC, Pletnikova O, Turchinovich A, Routenberg DA, Witwer KW. Huang Y, et al. Interdiscip Med. 2023 Oct;1(4):e20230016. doi: 10.1002/INMD.20230016. Epub 2023 Aug 15. Interdiscip Med. 2023. PMID: 38089920 Free PMC article. - Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response.
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A. Bachiller S, et al. Front Cell Neurosci. 2018 Dec 18;12:488. doi: 10.3389/fncel.2018.00488. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30618635 Free PMC article. Review. - Development and sensory experience dependent regulation of microglia in barrel cortex.
Kalambogias J, Chen CC, Khan S, Son T, Wercberger R, Headlam C, Lin C, Brumberg JC. Kalambogias J, et al. J Comp Neurol. 2020 Mar 1;528(4):559-573. doi: 10.1002/cne.24771. Epub 2019 Oct 18. J Comp Neurol. 2020. PMID: 31502243 Free PMC article. - Age Influences Microglial Activation After Cuprizone-Induced Demyelination.
Klein B, Mrowetz H, Barker CM, Lange S, Rivera FJ, Aigner L. Klein B, et al. Front Aging Neurosci. 2018 Sep 20;10:278. doi: 10.3389/fnagi.2018.00278. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30297998 Free PMC article.
References
- Alliot F., Godin I., Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Developmental Brain Research. 1999;117:145–152. - PubMed
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. - PubMed
- Ando S., Tanaka Y., Toyoda Y., Kon K. Turnover of myelin lipids in aging brain. Neurochemical Research. 2003;28:5–13. - PubMed
- Barrientos R.M., Higgins E.A., Biedenkapp J.C., Sprunger D.B., Wright-Hardesty K.J., Watkins L.R., Rudy J.W., Maier S.F. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiology of Aging. 2006;27:723–732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials