Long noncoding RNA-mediated anti-apoptotic activity in murine erythroid terminal differentiation - PubMed (original) (raw)
Long noncoding RNA-mediated anti-apoptotic activity in murine erythroid terminal differentiation
Wenqian Hu et al. Genes Dev. 2011.
Abstract
Long noncoding RNAs (lncRNAs) are differentially expressed under both normal and pathological conditions, implying that they may play important biological functions. Here we examined the expression of lncRNAs during erythropoiesis and identified an erythroid-specific lncRNA with anti-apoptotic activity. Inhibition of this lncRNA blocks erythroid differentiation and promotes apoptosis. Conversely, ectopic expression of this lncRNA can inhibit apoptosis in mouse erythroid cells. This lncRNA represses expression of Pycard, a proapoptotic gene, explaining in part the inhibition of programmed cell death. These findings reveal a novel layer of regulation of cell differentiation and apoptosis by a lncRNA.
© 2011 by Cold Spring Harbor Laboratory Press
Figures
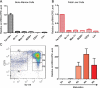
Figure 1.
LincRNA-EPS is highly specific to the terminal differentiating erythroid cells. Total nucleated mouse bone marrow cells (A) or fetal liver cells (B) were fractionated into erythroid cells (Ter119+), granulocytes (Gr-1+), monocytes (Mac-1+), B cells (B220+), T cells (CD3+), and lineage-depleted cells (Lin−) using antibodies to cell surface antigens and magnetic-assisted cell sorting (MACS), followed by total RNA extraction, reverse transcription, and quantitative PCR. The level of LincRNA-EPS in each fraction was normalized to that of 18S rRNA, and the normalized LincRNA-EPS level in Ter119+ erythroid cells was set as 1. (C) Total mouse fetal (E14.5) liver cells were fractionated into R1–R5 based on the expression levels of Ter119 and CD71; cells in each fraction were collected by fluorescence-activated cell sorting (FACS). Then, LincRNA-EPS levels were quantified by real-time RT–PCR and normalized to 18S rRNA; the normalized LincRNA-EPS level in R1 was set as 1. This quantification was based on three independent experiments, with the mean and the standard error of the mean indicated on the panels.
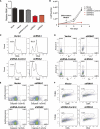
Figure 2.
Inhibition of LincRNA-EPS leads to apoptosis during terminal differentiation of erythroid cells. (A) LincRNA-EPS was knocked down by shRNAs encoded by retroviral vectors. Mouse fetal liver Lin− cells were transduced by retroviral vectors and cultured in maintenance medium for 24 h to allow shRNA expression and then switched to differentiation medium. The LincRNA-EPS level was determined on transduced cells (GFP+) at 24 h in differentiation medium by real-time RT–PCR and normalized to the level of 18S rRNA; the normalized LincRNA-EPS level in mock-treated cells was set as 1. shRNA1 and shRNA2 target different regions on LincRNA-EPS, and shRNA targeted to firefly luciferase was used as an shRNA control. (B) Growth curves of Lin− cells mock-treated or transduced by vector control, shRNA control, LincRNA-EPS shRNA1, or LincRNA-EPS shRNA2. The cell number at each time point is the average of three measurements. The cell cycle status (C) and apoptotic status determined by Annexin-V staining (D), caspase3 activity measurement (E), and TUNEL assay (F) were measured by flow cytometry at 24 h in differentiation medium. The DNA content in the cell cycle analysis (C) and TUNEL assay (F) was determined by Hoechst33342 and propidium iodide staining, respectively. The apoptosis analyses shown here are representative of three independent experiments.
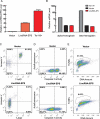
Figure 3.
Ectopic expression of LincRNA-EPS protects mouse erythroid progenitors from apoptosis induced by Epo deprivation. Lin− mouse fetal liver cells were transduced by retroviral vectors and maintained in maintenance medium for 40–48 h. The LincRNA-EPS levels (A) and hemoglobin mRNAs level (B) in transduced (GFP+) cells were quantified by real-time RT–PCR and normalized to 18S rRNA; the RNA level in the empty vector transduced cells was set as 1. Ter119+ cells were isolated by MACS from total nucleated fetal liver cells. Cell apoptosis was assayed by flow cytometry using Annexin-V staining (C), caspase 3 activity staining (D), and TUNEL assay (E). The top panels indicate the apoptotic state in the empty vector transduced Lin− cells, and the bottom panels indicate the apoptotic state in Lin− cells ectopically expressing LincRNA-EPS. The apoptosis analyses shown here are representative of three independent experiments.
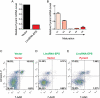
Figure 4.
Pycard is one functional target of LincRNA-EPS. (A) Relative Pycard mRNA level in Lin− cells transduced by the empty vector or the vector with LincRNA-EPS and cultured in maintenance medium. (B) Expression pattern of Pycard mRNA in R1–R5 fetal liver cells. The mRNA level was normalized to 18S rRNA, and the normalized mRNA level in R1 was set as 1. (C–E) Apoptotic state of 40-h Epo-starved Lin− cells doubly transduced with LincRNA-EPS ectopically expressed from a GFP-containing vector and Pycard overexpressed from a DsRed-expressing vector. The apoptosis analyses shown here are representative of three independent experiments.
Comment in
- A new 'Linc' between noncoding RNAs and blood development.
Paralkar VR, Weiss MJ. Paralkar VR, et al. Genes Dev. 2011 Dec 15;25(24):2555-8. doi: 10.1101/gad.183020.111. Genes Dev. 2011. PMID: 22190456 Free PMC article.
Similar articles
- A new 'Linc' between noncoding RNAs and blood development.
Paralkar VR, Weiss MJ. Paralkar VR, et al. Genes Dev. 2011 Dec 15;25(24):2555-8. doi: 10.1101/gad.183020.111. Genes Dev. 2011. PMID: 22190456 Free PMC article. - Shlnc-EC6 regulates murine erythroid enucleation by Rac1-PIP5K pathway.
Wang C, Wu X, Shen F, Li Y, Zhang Y, Yu D. Wang C, et al. Dev Growth Differ. 2015 Aug;57(6):466-473. doi: 10.1111/dgd.12225. Epub 2015 Jun 22. Dev Growth Differ. 2015. PMID: 26098172 - Novel roles for KLF1 in erythropoiesis revealed by mRNA-seq.
Tallack MR, Magor GW, Dartigues B, Sun L, Huang S, Fittock JM, Fry SV, Glazov EA, Bailey TL, Perkins AC. Tallack MR, et al. Genome Res. 2012 Dec;22(12):2385-98. doi: 10.1101/gr.135707.111. Epub 2012 Jul 26. Genome Res. 2012. PMID: 22835905 Free PMC article. - KLF1 directly coordinates almost all aspects of terminal erythroid differentiation.
Tallack MR, Perkins AC. Tallack MR, et al. IUBMB Life. 2010 Dec;62(12):886-90. doi: 10.1002/iub.404. IUBMB Life. 2010. PMID: 21190291 Review. - Primitive erythropoiesis in the mammalian embryo.
Palis J, Malik J, McGrath KE, Kingsley PD. Palis J, et al. Int J Dev Biol. 2010;54(6-7):1011-8. doi: 10.1387/ijdb.093056jp. Int J Dev Biol. 2010. PMID: 20711979 Review.
Cited by
- The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus.
Gomez JA, Wapinski OL, Yang YW, Bureau JF, Gopinath S, Monack DM, Chang HY, Brahic M, Kirkegaard K. Gomez JA, et al. Cell. 2013 Feb 14;152(4):743-54. doi: 10.1016/j.cell.2013.01.015. Cell. 2013. PMID: 23415224 Free PMC article. - Long non-coding RNAs and MYC association in hematological malignancies.
Benetatos L, Benetatou A, Vartholomatos G. Benetatos L, et al. Ann Hematol. 2020 Oct;99(10):2231-2242. doi: 10.1007/s00277-020-04166-4. Epub 2020 Jul 4. Ann Hematol. 2020. PMID: 32621182 Review. - Establishment of a cell-type-specific genetic network by the mediator complex component Med1.
Pope NJ, Bresnick EH. Pope NJ, et al. Mol Cell Biol. 2013 May;33(10):1938-55. doi: 10.1128/MCB.00141-13. Epub 2013 Mar 4. Mol Cell Biol. 2013. PMID: 23459945 Free PMC article. - Long non-coding RNAs in haematological malignancies.
Garitano-Trojaola A, Agirre X, Prósper F, Fortes P. Garitano-Trojaola A, et al. Int J Mol Sci. 2013 Jul 24;14(8):15386-422. doi: 10.3390/ijms140815386. Int J Mol Sci. 2013. PMID: 23887658 Free PMC article. Review. - Advances in long noncoding RNAs: identification, structure prediction and function annotation.
Guo X, Gao L, Wang Y, Chiu DK, Wang T, Deng Y. Guo X, et al. Brief Funct Genomics. 2016 Jan;15(1):38-46. doi: 10.1093/bfgp/elv022. Epub 2015 Jun 13. Brief Funct Genomics. 2016. PMID: 26072035 Free PMC article. Review.
References
- Amaral PP, Dinger ME, Mercer TR, Mattick JS 2008. The eukaryotic genome as an RNA machine. Science 319: 1787–1789 - PubMed
- Belloc F, Dumain P, Boisseau MR, Jalloustre C, Reiffers J, Bernard P, Lacombe F 1994. A flow cytometric method using Hoechst 33342 and propidium iodide for simultaneous cell cycle analysis and apoptosis determination in unfixed cells. Cytometry 17: 59–65 - PubMed
- Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al. 2005. The transcriptional landscape of the mammalian genome. Science 309: 1559–1563 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK068348/DK/NIDDK NIH HHS/United States
- R56 DK068348/DK/NIDDK NIH HHS/United States
- R01 DK068348/DK/NIDDK NIH HHS/United States
- 5P01 HL066105/HL/NHLBI NIH HHS/United States
- P01 HL066105/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous