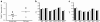Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets - PubMed (original) (raw)
Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets
C Dai et al. Diabetologia. 2012 Mar.
Abstract
Aims/hypothesis: Our understanding of the transcription factors that control the development and function of rodent islet beta cells is advancing rapidly, yet less is known of the role they play in similar processes in human islets.
Methods: To characterise the abundance and regulation of key proteins involved in glucose-regulated insulin secretion in human islets, we examined the expression of MAFA, MAFB, GLUT2 (also known as SLC2A2), βGK (also known as GCK) and PDX1 in isolated, highly purified human islets with an intact insulin secretory pattern. We also assessed these features in islets from two different mouse strains (C57BL/6J and FVB).
Results: Compared with mouse islets, human islets secreted more insulin at baseline glucose (5.6 mmol/l), but less upon stimulation with high glucose (16.7 mmol/l) or high glucose plus 3-isobutyl-1-methyl-xanthine. Human islets had relatively more MAFB than PDX1 mRNA, while mouse islets had relatively more Pdx1 than Mafb mRNA. However, v-maf musculoaponeurotic fibrosarcoma oncogene homologue (MAF) B protein was found in human islet alpha and beta cells. This is unusual as this regulator is only produced in islet alpha cells in adult mice. The expression of insulin, MAFA, βGK and PDX1 was not glucose-regulated in human islets with an intact insulin secretory pattern.
Conclusions/interpretation: Our results suggest that human islets have a distinctive distribution and function of key regulators of the glucose-stimulated insulin secretion pathway, emphasising the urgent need to understand the processes that regulate human islet beta cell function.
Figures
Fig. 1
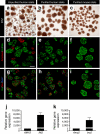
Human islets were isolated by handpicking under microscopic guidance. a An example of a human islet preparation received from an islet isolation facility and stained by dithizone (DTZ) before and (b) after the handpicking procedure. While this example was one of the more impure preparations studied (stated purity 50%), all islet preparations contained ductal and acinar fragments. During culture, acinar fragments rounded up and became similar in size and shape to human islets. These acinar structures could only be distinguished from islets by the lighter brown colour of human islets noted by experienced observers. In contrast, isolated mouse islets were much easier to distinguish from acinar tissue fragments and could readily be identified for handpicking. c An example of mouse islets stained by DTZ after handpicking. Scale bar (a–c) 100 μm. To further evaluate the purity of handpicked islets (d–f), selected islet preparations were processed for cryosections and labelled for insulin (Ins, green), glucagon (Glu, green) and α-amylase (red). The islet cell composition of human and mouse islets was similar to that observed in a previous report [3]. Note (d) that the size of some acinar fragments (amylase-positive) is similar to that of islets. Adjacent sections (g–i) were labelled for insulin (green), glucagon (red) and somatostatin (Som, blue). Note the difference in islet cell distribution between human and mouse islets. Scale bar (d–i) 100 μm. j High enrichment of human (n = 6) and (k) FVB mouse islet preparations (n = 4) for beta cells vs acinar cells was demonstrated by quantitative RT-PCR
Fig. 2
Assessment of cell viability in human and mouse islet preparations. a The numbers of TUNEL-positive beta cells in representative human (black circles, n = 9) and mouse FVB (white squares, n = 4) islet preparations were determined. Analysis was based on counting approximately 3,000 and 1,200 beta cells per human and mouse islet sample, respectively; p = 0.0673. b The expression profile of apoptosis-related genes as labelled in human (n = 6) and (c) FVB mouse islets (n = 5) cultured in 5 (black bars) or 11 mmol/l (white bars) glucose. BCLX/Bclx, also known as BCL2L1/Bcl2l1
Fig. 3
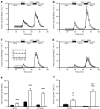
Insulin secretion in human and mouse islets. a Dynamic glucose-regulated insulin secretory characteristics of perifused human islets (n = 24), (b) FVB mouse islets (n = 9), (c) C57BL/6J mouse islets (n = 8) and (d) shipped C57BL/6J islets. The insulin concentration was determined by radioimmunoassay [19]. Note that the scale for the _y_-axis is different for human islets to make the insulin secretory pattern more readily visible. The amount of insulin secreted was normalised to IEQ, but it should be realised that islet cell composition in humans is different to that in mice [3]. Based on our experience with mouse and human islet preparations, our rationale for normalising insulin to IEQ is described above (Methods). ESM Fig. 3 shows secreted insulin normalised to islet insulin content. Here (a–d), the isolated human and mouse islets were precultured in 5 mmol/l glucose for 72 and 48 h, respectively. Human islets (a) showed a higher insulin output at basal 5.6 mmol/l glucose (G5.6) than mouse islets (b–d), and a lower stimulation index at 16.7 mmol/l glucose (G16.7) or 16.7 mmol/l glucose + 100 μmol/l IBMX (G16.7 + IBMX). The insert (c) shows basal secretion by human (black symbols), and FVB and C57BL/6J (white symbols) islets, which in the latter was similar and appears to overlap. An additional 24 h of culture of mouse islets did not change the magnitude of the perifusion response; dynamic GSIS in mouse islets was similar at 48 and 72 h of culture (data not shown). d To assess the effects of shipping on islet function, C57BL/6J mouse islets were isolated at Vanderbilt (n = 5 islet isolations; three separate shipments) and cultured for 24 h. Identical islet aliquots were either cultured in RPMI + 10% FBS (5 mmol/l glucose) or shipped by overnight courier to the University of Massachusetts using the shipping containers and conditions used by the Islet Cell Resource Centers and the Integrated Islet Distribution Network. Upon arrival at the University of Massachusetts, the shipping container was opened, labelled with a new shipping label and shipped back by the same overnight courier. Upon arrival at Vanderbilt, the cultured (black diamonds) and shipped (white diamonds) islet aliquots were simultaneously evaluated in the perifusion system (i.e. at approximately 72 h after isolation, with approximately 48 h of shipping time). Significantly, control C57BL/6J islets (cultured for approximately 72 h) and shipped islets had similar insulin secretory properties, as assessed by AUC per 100 IEQ; p = 0.368 for 16.7 mmol/l glucose peak; p = 0.322 for glucose + IBMX peak. e Integrated insulin secretion per 100 IEQ for mouse and human islets after stimulation with 16.7 mmol/l glucose (black bars) or 16.7 mmol/l glucose + 100 μmol/l IBMX (white bars); ***p < 0.001 for comparison with 16.7 mmol/l. One-way ANOVA was used for multiple group comparisons, with _p_ values as follows: 16.7 mmol/l _p_ < 0.001 for human vs FVB, _p_ > 0.05 for human vs C57 and p < 0.001 for FVB vs C57; 16.7 mmol/l glucose + 100 μmol/l IBMX p < 0.001 for human vs FVB and human vs C57, and p < 0.01 for FVB vs C57. f Static GSIS was measured in human (n = 8) and FVB mouse islets (n = 8) exposed to 5 (black bars) or 11 mmol/l (white bars) glucose for an additional 24 h. Human islets showed significantly higher basal insulin secretion than mouse, whereas GSIS in human islets increased only three fold vs 30-fold in mouse islets; *p < 0.05 and ***p < 0.001 for comparison with 5 mmol/l glucose; †† p < 0.01 and ††† p < 0.001 for comparison with human islets
Fig. 4
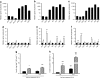
Human islet beta cell-enriched gene expression is not very responsive to stimulating glucose levels. a Human islets (n = 11), or FVB (b) and C57BL/6J (c) mouse islets (n = 4 each) were cultured for 72 or 48 h, respectively, after which total RNA was analysed by quantitative RT-PCR_._ mRNA levels for each of the measured islet-enriched gene products were normalised to four endogenous controls (i.e. 18S, actin, TFRC/Tfrc and TBP/Tbp in human and C57BL/6J mice, or 18 s rRNA in FVB mice) and expressed relative to βGK/βgk, which was set at 1. d Human (n = 11), (e) FVB mouse (n = 4) and (f) C57BL/6J mouse (n = 4) islets were cultured in 5 mmol/l glucose, and then in 5 (black bars) or 11 mmol/l (white bars) glucose for an additional 24 h before RNA analysis. The normalised mRNA level in 11 mmol/l glucose-cultured islets is expressed relative to 5 mmol/l glucose; *p < 0.05, **p < 0.01 and ***p < 0.001 compared with islets cultured in 5 mmol/l glucose. SOM/Som, also known as SST/Sst; GLU/Glu, also known as GCG/Gcg. g Human or (h) FVB mouse islets were cultured for 48 or 24 h, respectively, after isolation in 5 mmol/l glucose. Islets were then cultured in 5 (G5) or 11 mmol/l (G11) glucose for an additional 24 h (pretreatment) and subsequently challenged with 3 mmol/l (black bars) or 16.7 mmol/l (hatched bars) glucose for 30 min. Insulin released into the media was measured by RIA. Gene expression in selected mouse and human islet preparations was similar at 24 and 48 h of culture (data not shown). *p < 0.05 and ***p < 0.001 compared with response to 3 mmol/l glucose; ††† p < 0.001 compared with response of islets incubated in 5 mmol/l glucose for 24 h
Fig. 5
MAFA and PDX1 transcription factor mRNA levels are not glucose-regulated in cultured human islets. a Human (n = 15), (b) FVB (n = 4) and (c) C57BL/6J (n = 4) mouse islets were cultured for 72 h (human) or 48 h (mice), respectively. MAFA/Mafa, MAFB/Mafb and PDX1/Pdx1 mRNA levels were then quantified by RT-PCR and expressed relative to MAFA/Mafa. d Human (n = 11), (e) FVB (n = 4) and (f) C57BL/6J (n = 4) mouse islets were first cultured for 48 h (human) or 24 h (mouse) in 5 mmol/l glucose, and then for 24 h in 5 (black bars) or 11 mmol/l (white bars) glucose before RNA analysis. The normalised amount of mRNA in 11 mmol/l glucose-cultured islets is expressed relative to 5 mmol/l glucose-cultured islets; *p < 0.05 and ***p < 0.001 compared with islets cultured in 5 mmol/l glucose
Fig. 6
MAFA, PDX1 and MAFB are present in the human islet beta cell population. The percentage of MAFA-, MAFB- or PDX1-producing alpha (glucagon, Glu), beta (insulin, Ins) and delta (somatostatin, Som) cells was determined from the immunohistochemical staining pattern. The intact human pancreas index (a, e, i, m, q) was 55% MAFA+ beta cells (n = 2,935 [total number of analysed cells]), 80% PDX1+ beta cells (n = 1,700), 9% MAFB+ beta cells (n = 1,567) and 40% MAFB+ alpha cells (n = 1,150). MAFA and PDX1 were produced in a larger fraction of beta cells in the mouse pancreas (c, g, k, o, s): 95% MAFA+ beta cells (n = 2,470), 100% PDX1+ beta cells (n = 1,516), 0.4% MAFB+ beta cells (n = 670) and 73% MAFB+ alpha cells (n = 327). e–h Arrows point to Ins+/MAFB+ cells, arrowheads indicate Ins−/MAFB+ cells. Scale bar (a) 50 μm applies to all other panels (b–t). Because, in human islets, beta and non-beta cells are more intermingled, two criteria were used for cell-counting purposes to ensure cell identity. First, an islet cell was deemed positive for nuclear factor or TUNEL only when at least 75% of the nucleus was surrounded by the cytoplasm labelled for a given hormone. Second, if the first criterion was met and there was a gap between the nucleus and the cell cytoplasm, this cell was excluded from the analysis. Thus, it is possible that due to the stringency of our counting procedure, counts of cells doubly positive for a hormone and a transcription factor (especially in human islets) may have been slightly underestimated
Similar articles
- Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells.
Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Matsuoka TA, et al. Mol Cell Biol. 2003 Sep;23(17):6049-62. doi: 10.1128/MCB.23.17.6049-6062.2003. Mol Cell Biol. 2003. PMID: 12917329 Free PMC article. - A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells.
Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. Nishimura W, et al. Dev Biol. 2006 May 15;293(2):526-39. doi: 10.1016/j.ydbio.2006.02.028. Epub 2006 Apr 3. Dev Biol. 2006. PMID: 16580660 Free PMC article. - Hes3 is expressed in the adult pancreatic islet and regulates gene expression, cell growth, and insulin release.
Masjkur J, Arps-Forker C, Poser SW, Nikolakopoulou P, Toutouna L, Chenna R, Chavakis T, Chatzigeorgiou A, Chen LS, Dubrovska A, Choudhary P, Uphues I, Mark M, Bornstein SR, Androutsellis-Theotokis A. Masjkur J, et al. J Biol Chem. 2014 Dec 19;289(51):35503-16. doi: 10.1074/jbc.M114.590687. Epub 2014 Nov 4. J Biol Chem. 2014. PMID: 25371201 Free PMC article. - MafA and MafB activity in pancreatic β cells.
Hang Y, Stein R. Hang Y, et al. Trends Endocrinol Metab. 2011 Sep;22(9):364-73. doi: 10.1016/j.tem.2011.05.003. Epub 2011 Jun 28. Trends Endocrinol Metab. 2011. PMID: 21719305 Free PMC article. Review. - PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.
Zhu Y, Liu Q, Zhou Z, Ikeda Y. Zhu Y, et al. Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
Cited by
- Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles.
Kayton NS, Poffenberger G, Henske J, Dai C, Thompson C, Aramandla R, Shostak A, Nicholson W, Brissova M, Bush WS, Powers AC. Kayton NS, et al. Am J Physiol Endocrinol Metab. 2015 Apr 1;308(7):E592-602. doi: 10.1152/ajpendo.00437.2014. Epub 2015 Feb 3. Am J Physiol Endocrinol Metab. 2015. PMID: 25648831 Free PMC article. - Human islets expressing HNF1A variant have defective β cell transcriptional regulatory networks.
Haliyur R, Tong X, Sanyoura M, Shrestha S, Lindner J, Saunders DC, Aramandla R, Poffenberger G, Redick SD, Bottino R, Prasad N, Levy SE, Blind RD, Harlan DM, Philipson LH, Stein RW, Brissova M, Powers AC. Haliyur R, et al. J Clin Invest. 2019 Jan 2;129(1):246-251. doi: 10.1172/JCI121994. Epub 2018 Dec 3. J Clin Invest. 2019. PMID: 30507613 Free PMC article. - (Re-)Viewing Role of Intracellular Glucose Beyond Extracellular Regulation of Glucose-Stimulated Insulin Secretion by Pancreatic Cells.
Firdos, Pramanik T, Verma P, Mittal A. Firdos, et al. ACS Omega. 2024 Feb 29;9(10):11755-11768. doi: 10.1021/acsomega.3c09171. eCollection 2024 Mar 12. ACS Omega. 2024. PMID: 38496986 Free PMC article. - Human Islets Have Fewer Blood Vessels than Mouse Islets and the Density of Islet Vascular Structures Is Increased in Type 2 Diabetes.
Brissova M, Shostak A, Fligner CL, Revetta FL, Washington MK, Powers AC, Hull RL. Brissova M, et al. J Histochem Cytochem. 2015 Aug;63(8):637-45. doi: 10.1369/0022155415573324. J Histochem Cytochem. 2015. PMID: 26216139 Free PMC article. - Mechanisms of β-cell functional adaptation to changes in workload.
Wortham M, Sander M. Wortham M, et al. Diabetes Obes Metab. 2016 Sep;18 Suppl 1(Suppl 1):78-86. doi: 10.1111/dom.12729. Diabetes Obes Metab. 2016. PMID: 27615135 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK068751/DK/NIDDK NIH HHS/United States
- U19 DK042502/DK/NIDDK NIH HHS/United States
- DK69603/DK/NIDDK NIH HHS/United States
- R21 DK062641/DK/NIDDK NIH HHS/United States
- R21 DK068854/DK/NIDDK NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- DK66636/DK/NIDDK NIH HHS/United States
- U01 DK089572/DK/NIDDK NIH HHS/United States
- R33 DK066636/DK/NIDDK NIH HHS/United States
- DK72473/DK/NIDDK NIH HHS/United States
- DK89572/DK/NIDDK NIH HHS/United States
- R01 DK069603/DK/NIDDK NIH HHS/United States
- DK68854/DK/NIDDK NIH HHS/United States
- P01 DK042502/DK/NIDDK NIH HHS/United States
- R01 DK090570/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- I01 BX000666/BX/BLRD VA/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- DK68751/DK/NIDDK NIH HHS/United States
- R01 DK068764/DK/NIDDK NIH HHS/United States
- DK42502/DK/NIDDK NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- DK63439/DK/NIDDK NIH HHS/United States
- DK62641/DK/NIDDK NIH HHS/United States
- R21 DK066636/DK/NIDDK NIH HHS/United States
- R21 DK063439/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous