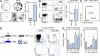Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis - PubMed (original) (raw)
. 2012 Jan 16;209(1):123-37.
doi: 10.1084/jem.20111009. Epub 2012 Jan 2.
Philipp J Rauch, Takuya Ueno, Rostic Gorbatov, Brett Marinelli, Won Woo Lee, Partha Dutta, Ying Wei, Clinton Robbins, Yoshiko Iwamoto, Brena Sena, Aleksey Chudnovskiy, Peter Panizzi, Edmund Keliher, John M Higgins, Peter Libby, Michael A Moskowitz, Mikael J Pittet, Filip K Swirski, Ralph Weissleder, Matthias Nahrendorf
Affiliations
- PMID: 22213805
- PMCID: PMC3260875
- DOI: 10.1084/jem.20111009
Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis
Florian Leuschner et al. J Exp Med. 2012.
Abstract
Monocytes (Mo) and macrophages (MΦ) are emerging therapeutic targets in malignant, cardiovascular, and autoimmune disorders. Targeting of Mo/MΦ and their effector functions without compromising innate immunity's critical defense mechanisms first requires addressing gaps in knowledge about the life cycle of these cells. Here we studied the source, tissue kinetics, and clearance of Mo/MΦ in murine myocardial infarction, a model of acute inflammation after ischemic injury. We found that a) Mo tissue residence time was surprisingly short (20 h); b) Mo recruitment rates were consistently high even days after initiation of inflammation; c) the sustained need of newly made Mo was fostered by extramedullary monocytopoiesis in the spleen; d) splenic monocytopoiesis was regulated by IL-1β; and e) the balance of cell recruitment and local death shifted during resolution of inflammation. Depending on the experimental approach, we measured a 24 h Mo/MΦ exit rate from infarct tissue between 5 and 13% of the tissue cell population. Exited cells were most numerous in the blood, liver, and spleen. Abrogation of extramedullary monocytopoiesis proved deleterious for infarct healing and accelerated the evolution of heart failure. We also detected rapid Mo kinetics in mice with stroke. These findings expand our knowledge of Mo/MΦ flux in acute inflammation and provide the groundwork for novel anti-inflammatory strategies for treating heart failure.
Figures
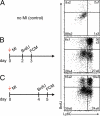
Figure 1.
Monocyte infarct residence time after ischemia. Cell tissue kinetics were studied using flow cytometric analysis (FCM) of heart transplants and BrdU pulse experiments. (A) Schematic set-up of the experiment (n = 16; experiment was performed four times). (B) Dot plots of FCM analysis showing the contribution of donor- and recipient-derived Mo in the infarcted heart at 6, 12, and 24 h after transplantation of the heart. (C) Number of Mo retrieved from digested infarcts on day 3 to 6 after MI by FCM. (D) Fitting of donor Mo (y-axis) over time (x-axis). Red circles indicate individual data, the gray line represents the fit described by the equation. (E) Flow cytometric DAPI staining of Mo in the infarct on day 3 after coronary ligation. (F) FCM analysis of the infarct 24 h after BrdU pulse. Dot plots are gated on Mo. (G) Fold-change in BrdU+ Mo and lineage+ cells in the heart after MI. Mean ± SEM (n = 3–10 per group, experiment was repeated twice). *, P < 0.05.
Figure 2.
Kinetics of Mo subsets after MI. (A) FCM of BrdU pulse experiment in naive mice on day 3 after MI (B) and on day 5 after MI (C). Dot plots show Mo identified as CD11bhigh (CD90/B220/CD49b/NK1.1/Ly-6G)low (F4/80/CD11c)low, including separation into subsets using Ly-6C. Mean ± SEM (n = 4–6 per group, experiment was repeated twice).
Figure 3.
Mo clearance. Cell exit and local death were studied as major contributors to cell clearance from the site of inflammation. (A) Bar graph shows number of exited CD45.2+ Mo in organs based on FCM; pie chart illustrates percentage of exited myeloid cells normalized to the number in the infarct (n = 5). (B) Left bar graph shows excited cell number in organs calculated using alternative scintillation data; pie chart illustrates the exit rate. The right bar graph reflects decay-corrected photon counts of the graft before and 24 h after heart transplantation. Experiment was done in duplicate, data are mean ± SEM. (C) Immunofluorescence of infarct tissue (DAPI, blue; TUNEL, green; CD11b, red). Pie charts indicate frequency of TUNEL signal in CD11b+ cells (arrows; n = 5 per group). (D) Analysis of cell death by DAPI FCM staining in the heart (n = 4 per group). (E) In vitro time lapse microscopy of representative splenic Mo to determine timing of cell death signals. Experiment was done in duplicates.
Figure 4.
The spleen is a major source of Mo during acute inflammation. (A) Gate in dot plots of infarcted hearts shows myeloid cells on day 6 after MI (SPX indicates splenectomy 24 h before analysis; Lin indicates staining for lineage markers). Bar graphs enumerate total number of Mo in the heart, bone marrow, and blood (n = 6–9 per group, from three independent experiments). (B) Histograms gated on Mo in the heart. Bar graph shows the total number of BrdU+ Mo. Mean ± SEM (n = 4–6 per group, experiment performed twice). *, P < 0.05.
Figure 5.
MI induces extramedullary monocytopoiesis in the spleen. (A) CFU assay of splenocytes from naive mice and from mice 6 d after MI (n = 3 per group, experiment performed twice). Top images show representative scans of the culture plate, bottom images show magnifications of colonies. (right) Bar graph enumerates colonies in cultures. (B) Representative dot plots from spleen and enumeration of splenic MDPs. lin* indicates lineage for myeloid progenitor staining as described in the Materials and methods section (n = 6–9 per group from three independent experiments). (C) Cell cycle analysis for splenic MDPs in mice after MI (n = 3 per group from one experiment). (D) Adoptive transfer of GMPs on day 3 after MI. CD45.2+ cells were transferred into infarcted CD45.1+ mice, which were analyzed 3 d later. Dot plots show adoptively transferred precursors in the splenic pool and the infarcted myocardium (n = 6 from one experiment). (E) Ly-6Chigh and Ly-6Clow Mo in the spleen after MI. Mean ± SEM (n = 4–10 per group from 4 independent experiments). *, P < 0.05.
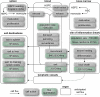
Figure 6.
Current systemic MPS flux model in subacute MI in the mouse. The model was populated with numbers and rates obtained in mice with coronary ligation and is a first attempt at a more systematic visualization of system-wide cell kinetics in acute inflammation.
Figure 7.
Serial in vivo imaging reveals key function of splenic Mo/MΦ in infarct healing. Study groups: MI and no SPX (top row), MI and SPX on d0 (middle), and MI and SPX on d3 after MI (bottom). Three-channel FMT-CT was done on day 5 after MI. Bar graphs on the bottom show fluorescence signal in the infarct in respective channels. MR images from day 1 (inset) and day 21 (full size) are shown on the right, with bar graphs on the bottom. Arrows indicate gadolinium-DTPA enhanced infarct. Mean ± SEM (n = 8–12 per group from two experiments). *, P < 0.05.
Figure 8.
Histological biomarkers of infarct healing after splenectomy. Experimental groups: MI and no SPX (top row), MI and SPX on d0 (middle), and MI and SPX on d3 after MI (bottom). IHC staining for myeloid cells (CD11b), MΦ (MAC-3), neo-vascularization (CD31), and collagen deposition (Masson trichrome, picrosirius red [PSR], and collagen I). ROI, region of interest. Mean ± SEM (n = 5 per group from one experiment). *, P < 0.05.
Figure 9.
Rapid Mo kinetics and supply of splenic Mo in stroke. (A) Dot plots from brain tissue gated on CD11b+ and lineage− cells 24 h after BrdU pulse. Mean ± SEM (n = 4–5 per group; P < 0.05 stroke vs. no stroke). (B) Pie chart illustrates turnover of Ly-6Chigh Mo in the brain on day 2 after stroke. (C) Number of Mo in the spleen after stroke. Dot plots are gated on CD11b+ lin− cells and compare naive control mice to mice 2 d after stroke. Bar graph shows total Mo in the spleen. Mean ± SEM (*, P < 0.05; n = 4–5 per group from one experiment). (D) Increased MDP on day 10 after stroke in the spleen. Mean ± SEM (*, P < 0.05, n = 4–5 per group from one experiment).
Figure 10.
IL-1β is a key cytokine for splenic monocytopoiesis. (A) rtPCR of IL-1β mRNA expression in the spleen throughout the first week after MI (n = 6–7 per group; *, P < 0.05). IL-1β protein by ELISA in the spleen on day 6 after MI (n = 4 per group; *, P < 0.05). (B) Cultured spleen cells from WT and IL-1R−/− mice colony forming capacity on day 6 after MI. Experiment was done in duplicates. (C) Enumeration after FCM analysis on day 6 after MI of splenic MDPs (left), BrdU+ Mo in the spleen (middle), and in the heart (right) of IL-1R−/− mice compared with WT. Mean ± SEM (n = 5 per group; *, P < 0.05). (D) Setup of the experiment: Adoptive transfer of CD45.2+ GMPs into infarcted CD45.1+ mice 1 d after MI. Analysis was performed 5 d later, comparing the capacity of transferred cells to generate Mo in the presence (WT) or absence (IL-1−/−) of the IL-1R. (E) Dot plots from spleens are gated on Mo, identified as CD11b+, lineage−, and CD11c−. Mean percentage ± SEM (n = 4 recipients per group). (F) Enumeration of CD45.2+ Mo in the spleens of CD45.1+ recipients 6 d after MI and 5 d after adoptive transfer. Mean ± SEM (n = 4 per group; *, P < 0.05).
Similar articles
- Proliferation of Ly6C+ monocytes/macrophages contributes to their accumulation in mouse skin wounds.
Pang J, Urao N, Koh TJ. Pang J, et al. J Leukoc Biol. 2020 Apr;107(4):551-560. doi: 10.1002/JLB.3HI1119-389RRRR. Epub 2019 Nov 28. J Leukoc Biol. 2020. PMID: 31777992 Free PMC article. - Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain.
Garcia-Bonilla L, Faraco G, Moore J, Murphy M, Racchumi G, Srinivasan J, Brea D, Iadecola C, Anrather J. Garcia-Bonilla L, et al. J Neuroinflammation. 2016 Nov 4;13(1):285. doi: 10.1186/s12974-016-0750-0. J Neuroinflammation. 2016. PMID: 27814740 Free PMC article. - Ly6CLo Monocyte/Macrophages are Essential for Thrombus Resolution in a Murine Model of Venous Thrombosis.
Kimball AS, Obi AT, Luke CE, Dowling AR, Cai Q, Adili R, Jankowski H, Schaller M, Holinstadt M, Jaffer FA, Kunkel SL, Gallagher KA, Henke PK. Kimball AS, et al. Thromb Haemost. 2020 Feb;120(2):289-299. doi: 10.1055/s-0039-3400959. Epub 2019 Dec 30. Thromb Haemost. 2020. PMID: 31887775 Free PMC article. - Monocytes in myocardial infarction.
Dutta P, Nahrendorf M. Dutta P, et al. Arterioscler Thromb Vasc Biol. 2015 May;35(5):1066-70. doi: 10.1161/ATVBAHA.114.304652. Epub 2015 Mar 19. Arterioscler Thromb Vasc Biol. 2015. PMID: 25792449 Free PMC article. Review. - The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: a reappraisal.
Katsiari CG, Liossis SN, Sfikakis PP. Katsiari CG, et al. Semin Arthritis Rheum. 2010 Jun;39(6):491-503. doi: 10.1016/j.semarthrit.2008.11.002. Epub 2009 Jan 15. Semin Arthritis Rheum. 2010. PMID: 19147182 Review.
Cited by
- Electroimmunology and cardiac arrhythmia.
Grune J, Yamazoe M, Nahrendorf M. Grune J, et al. Nat Rev Cardiol. 2021 Aug;18(8):547-564. doi: 10.1038/s41569-021-00520-9. Epub 2021 Mar 2. Nat Rev Cardiol. 2021. PMID: 33654273 Free PMC article. Review. - Left Coronary Artery Ligation: A Surgical Murine Model of Myocardial Infarction.
Johny E, Dutta P. Johny E, et al. J Vis Exp. 2022 Aug 9;(186):10.3791/64387. doi: 10.3791/64387. J Vis Exp. 2022. PMID: 36036590 Free PMC article. - Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior.
Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Wohleb ES, et al. Front Neurosci. 2015 Jan 21;8:447. doi: 10.3389/fnins.2014.00447. eCollection 2014. Front Neurosci. 2015. PMID: 25653581 Free PMC article. Review. - Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function.
Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Kain V, et al. J Mol Cell Cardiol. 2015 Jul;84:24-35. doi: 10.1016/j.yjmcc.2015.04.003. Epub 2015 Apr 11. J Mol Cell Cardiol. 2015. PMID: 25870158 Free PMC article. - Administration of cardiac mesenchymal cells modulates innate immunity in the acute phase of myocardial infarction in mice.
Kang Y, Nasr M, Guo Y, Uchida S, Weirick T, Li H, Kim J, Moore JB 4th, Muthusamy S, Bolli R, Wysoczynski M. Kang Y, et al. Sci Rep. 2020 Sep 8;10(1):14754. doi: 10.1038/s41598-020-71580-z. Sci Rep. 2020. PMID: 32901075 Free PMC article.
References
- Auffray C., Fogg D.K., Narni-Mancinelli E., Senechal B., Trouillet C., Saederup N., Leemput J., Bigot K., Campisi L., Abitbol M., et al. 2009. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J. Exp. Med. 206:595–606 10.1084/jem.20081385 - DOI - PMC - PubMed
- Brugger W., Möcklin W., Heimfeld S., Berenson R.J., Mertelsmann R., Kanz L. 1993. Ex vivo expansion of enriched peripheral blood CD34+ progenitor cells by stem cell factor, interleukin-1 beta (IL-1 beta), IL-6, IL-3, interferon-gamma, and erythropoietin. Blood. 81:2579–2584 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL095612/HL/NHLBI NIH HHS/United States
- R24 CA092782/CA/NCI NIH HHS/United States
- U01 HL080731/HL/NHLBI NIH HHS/United States
- HHSN268201000044C/HL/NHLBI NIH HHS/United States
- R01 HL096576/HL/NHLBI NIH HHS/United States
- R24-CA92782/CA/NCI NIH HHS/United States
- R01 HL095629/HL/NHLBI NIH HHS/United States
- UO1-HL080731/HL/NHLBI NIH HHS/United States
- R01 AI084880/AI/NIAID NIH HHS/United States
- R01HL095629/HL/NHLBI NIH HHS/United States
- R01HL096576/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials