Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration - PubMed (original) (raw)
Review
Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration
Zu-Hang Sheng et al. Nat Rev Neurosci. 2012.
Abstract
Mitochondria have a number of essential roles in neuronal function. Their complex mobility patterns within neurons are characterized by frequent changes in direction. Mobile mitochondria can become stationary or pause in regions that have a high metabolic demand and can move again rapidly in response to physiological changes. Defects in mitochondrial transport are implicated in the pathogenesis of several major neurological disorders. Research into the mechanisms that regulate mitochondrial transport is thus an important emerging frontier.
Figures
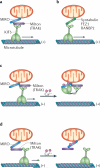
Figure 1. Mitochondrial transport in neurons
a | Neurons have three distinct functional and structural domains: a cell body (soma), a long axon and thick dendrites with many branches and elaborate dendritic arbors. Owing to the complex geometry of neurons, specialized mechanisms are required to transport mitochondria to their destinations and to ensure that mitochondria remain stationary in regions with a high demand for energy production and Ca2+ homeostatic capacity. The figure highlights the molecular mechanisms that are involved in transporting mitochondria to three specific neuronal locations: the presynaptic terminal, the axon and the dendritic spine. Long-range mitochondrial transport from the soma to distal axonal and dendritic regions depends on the polarity and organization of neuronal microtubules. Axonal microtubules are arranged so that their plus end (+) is directed distally and their minus end (−) is directed towards the soma. Thus, in axons, cytoplasmic dynein motors are responsible for returning mitochondria to the soma, whereas kinesin motors of the KIF5 family drive anterograde mitochondrial transport to distal axonal regions and synaptic terminals. For dendritic processes, where microtubules exhibit mixed polarity in proximal regions, kinesins and dynein motors can transport mitochondria in either the anterograde or the retrograde direction depending on the microtubule polarity. KIF5 motors link to the mitochondria that they transport via motor adaptors. Myosin motors probably mediate short-range movement in presynaptic terminals, growth cones and dendritic spines, where actin filaments form the major cytoskeletal architecture. Mobile mitochondria can also be recruited to stationary pools via dynamic interactions between the docking receptor syntaphilin and microtubules or via actin-based anchoring machinery. Mitochondrial docking mechanisms ensure that stationary mitochondria are adequately distributed within axons and at synapses. b | Mitochondrial movement and accumulation near a node of Ranvier in a peripheral nervous system myelinated axon. A high density of Na+ channels and Na+/K+ ATPases at the nodes of Ranvier is essential for myelinated axons to conduct high-velocity nerve impulses and to permit repetitive firing. Thus, regions near nodes of Ranvier have a high energy demand. KIF5 and dynein motors drive either anterograde or retrograde mitochondrial transport towards the node of Ranvier. Mobile mitochondria become stationary at the node and serve as energy sources to support Na+ channels and Na+/K+ ATPases.
Figure 2. KIF5-driven mitochondrial transport
a | Mitochondrial transport driven by kinesins of the KIF5 family requires the mitochondrial rho (MIRO)–Milton (or MIRO–TRAK) adaptor complex. MIRO is a mitochondrial outer membrane protein of the RHO GTPase family. In Drosophila melanogaster, Milton recruits KIF5 to mitochondria by binding to MIRO. In a similar way, TRAK1 and TRAK2 (mammalian Milton orthologues) can bind to MIRO1 and MIRO2 (mammalian orthologues of MIRO). In hippocampal neurons, the MIRO1–TRAK2 complex is an important regulator of mitochondrial trafficking. b | KIF5 also associates with mitochondria and mediates mitochondrial anterograde transport via syntabulin, a KIF5 adaptor that binds to mitochondria via its carboxy-terminal transmembrane domain. Fasciculation and elongation protein-ζ1 (FEZ1) and RAN-binding protein 2 (RANBP2) are additional kinesin adaptors that may contribute to mitochondrial transport. c | There are two proposed models of the role of MIRO in the regulation of mitochondrial mobility. MIRO contains two Ca2+-binding regions (EF-hand motifs), which allow it to regulate mitochondrial mobility in response to synaptic activity and Ca2+ signalling pathways. The C-terminus of the KIF5 motor attaches to mitochondria through an interaction with the MIRO–Milton (or MIRO–TRAK) complex in the absence of Ca2+, whereas its amino-terminal motor domain binds to microtubules and drives transport. In the ‘motor–MIRO binding’ model, Ca2+ binds to the EF hands of MIRO and induces the KIF5 motor domain to bind to MIRO instead of microtubules and thus prevents motor–microtubule engagement. d | Alternatively, in the ‘motor-releasing’ model, Ca2+ binding releases KIF5 from MIRO-bound mitochondria. Thus, Ca2+ influx following synaptic activity could cause mobile mitochondria to become stationary.
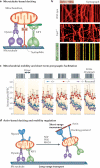
Figure 3. Mitochondrial docking and synaptic homeostasis
a | Microtubule-based docking. Mitochondria have complex mobility patterns that suggest that they are coupled to two opposing molecular motors, namely kinesin-1 (KIF5) and dynein, as well as to docking machinery. Whereas KIF5 motors are responsible for anterograde mitochondrial transport, cytoplasmic dynein motors are the driving force behind retrograde movement. Syntaphilin, a neuron-specific and axon-targeted protein that associates with the mitochondrial outer membrane, acts as an anchor for axonal mitochondria by binding to microtubules. b | Representative kymographs showing relative mitochondrial mobility in axons. The upper panel shows wild-type neurons transfected with the mitochondrial marker DsRed–Mito (red). The middle panel shows syntaphilin-deficient neurons transfected with DsRed–Mito. The lower panel shows wild-type neurons co-transfected with DsRed–Mito and green fluorescent protein (GFP)–syntaphilin (green). In kymographs, vertical lines represent stationary mitochondria, whereas slanted or curved lines indicate mobile ones. Overexpressing GFP–syntaphilin in wild-type neurons abolishes axonal mitochondrial transport (lower kymograph). Conversely, deleting the murine syntaphilin gene (Snph) results in a dramatic increase in the percentage of axonal mitochondria in dynamic mobile states (76 ± 20%) (middle kymograph; Supplementary information S2 (movie)) relative to wild-type neurons (36 ± 15%) (upper kymograph; Supplementary information S1 (movie)). c | Increased mitochondrial mobility in _Snph_−/− neurons induces short-term presynaptic facilitation during prolonged stimulation. A 20 Hz, 1 second stimulus train was delivered at 10-second intervals. Normalized excitatory postsynaptic current (EPSC) amplitudes were plotted against stimulus number. Persistent facilitation in synaptic responses occurred only in _Snph_−/− neurons (red circles). Reintroducing syntaphilin into the mutant presynaptic neurons (purple circles) eliminated the short-term presynaptic facilitation and fully rescued the wild-type phenotype (blue triangles). d | Schematic model of the transition from long-range, microtubule-based mitochondrial transport to myosin-driven short-range, actin-based movement and of subsequent stationary docking on actin through unknown docking and anchoring receptors. Actin-based docking is facilitated by nerve growth factor (NGF), phosphoinositide 3-kinase (PI3K) or RHOA signalling pathways. Part c is modified, with permission, from REF. © (2008) Elsevier.
Similar articles
- Regulation of mitochondrial transport in neurons.
Lin MY, Sheng ZH. Lin MY, et al. Exp Cell Res. 2015 May 15;334(1):35-44. doi: 10.1016/j.yexcr.2015.01.004. Epub 2015 Jan 19. Exp Cell Res. 2015. PMID: 25612908 Free PMC article. Review. - Mitochondrial trafficking and anchoring in neurons: New insight and implications.
Sheng ZH. Sheng ZH. J Cell Biol. 2014 Mar 31;204(7):1087-98. doi: 10.1083/jcb.201312123. J Cell Biol. 2014. PMID: 24687278 Free PMC article. Review. - Regulation and roles of mitophagy at synapses.
Palikaras K, Tavernarakis N. Palikaras K, et al. Mech Ageing Dev. 2020 Apr;187:111216. doi: 10.1016/j.mad.2020.111216. Epub 2020 Feb 19. Mech Ageing Dev. 2020. PMID: 32084458 Review. - Mitochondrial trafficking and morphology in healthy and injured neurons.
Chang DT, Reynolds IJ. Chang DT, et al. Prog Neurobiol. 2006 Dec;80(5):241-68. doi: 10.1016/j.pneurobio.2006.09.003. Epub 2006 Dec 26. Prog Neurobiol. 2006. PMID: 17188795 Review. - Regulation of axonal mitochondrial transport and its impact on synaptic transmission.
Cai Q, Davis ML, Sheng ZH. Cai Q, et al. Neurosci Res. 2011 May;70(1):9-15. doi: 10.1016/j.neures.2011.02.005. Epub 2011 Feb 23. Neurosci Res. 2011. PMID: 21352858 Free PMC article. Review.
Cited by
- Brain transcriptomic profiling reveals common alterations across neurodegenerative and psychiatric disorders.
Sadeghi I, Gispert JD, Palumbo E, Muñoz-Aguirre M, Wucher V, D'Argenio V, Santpere G, Navarro A, Guigo R, Vilor-Tejedor N. Sadeghi I, et al. Comput Struct Biotechnol J. 2022 Aug 19;20:4549-4561. doi: 10.1016/j.csbj.2022.08.037. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36090817 Free PMC article. - Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder.
Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A, Cacace AM, Zaczek R, Albright CF, Tseng G, Lewis DA. Arion D, et al. Mol Psychiatry. 2015 Nov;20(11):1397-405. doi: 10.1038/mp.2014.171. Epub 2015 Jan 6. Mol Psychiatry. 2015. PMID: 25560755 Free PMC article. - Pathogenesis of DJ-1/PARK7-Mediated Parkinson's Disease.
Skou LD, Johansen SK, Okarmus J, Meyer M. Skou LD, et al. Cells. 2024 Feb 6;13(4):296. doi: 10.3390/cells13040296. Cells. 2024. PMID: 38391909 Free PMC article. Review. - Diabetes Mellitus Induces Alzheimer's Disease Pathology: Histopathological Evidence from Animal Models.
Kimura N. Kimura N. Int J Mol Sci. 2016 Apr 5;17(4):503. doi: 10.3390/ijms17040503. Int J Mol Sci. 2016. PMID: 27058526 Free PMC article. Review. - Computational study of the motor neuron protein KIF5A to identify nsSNPs, bioactive compounds, and its key regulators.
Kumar R, Madhavan T, Ponnusamy K, Sohn H, Haider S. Kumar R, et al. Front Genet. 2023 Nov 10;14:1282234. doi: 10.3389/fgene.2023.1282234. eCollection 2023. Front Genet. 2023. PMID: 38028604 Free PMC article.
References
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol. Rev. 2000;80:315–360. - PubMed
- Verstreken P, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. [This paper shows that in D. melanogaster with mutant DRP1, the loss of mitochondria from neuromuscular junctions results in faster depletion of synaptic vesicles during prolonged pulse train stimulation owing to a specific defect in mobilizing reserve pool vesicles.] - PubMed
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical