Evolutionary context for understanding and manipulating plant responses to past, present and future atmospheric [CO2] - PubMed (original) (raw)
Review
Evolutionary context for understanding and manipulating plant responses to past, present and future atmospheric [CO2]
Andrew D B Leakey et al. Philos Trans R Soc Lond B Biol Sci. 2012.
Abstract
Variation in atmospheric [CO(2)] is a prominent feature of the environmental history over which vascular plants have evolved. Periods of falling and low [CO(2)] in the palaeo-record appear to have created selective pressure for important adaptations in modern plants. Today, rising [CO(2)] is a key component of anthropogenic global environmental change that will impact plants and the ecosystem goods and services they deliver. Currently, there is limited evidence that natural plant populations have evolved in response to contemporary increases in [CO(2)] in ways that increase plant productivity or fitness, and no evidence for incidental breeding of crop varieties to achieve greater yield enhancement from rising [CO(2)]. Evolutionary responses to elevated [CO(2)] have been studied by applying selection in controlled environments, quantitative genetics and trait-based approaches. Findings to date suggest that adaptive changes in plant traits in response to future [CO(2)] will not be consistently observed across species or environments and will not be large in magnitude compared with physiological and ecological responses to future [CO(2)]. This lack of evidence for strong evolutionary effects of elevated [CO(2)] is surprising, given the large effects of elevated [CO(2)] on plant phenotypes. New studies under more stressful, complex environmental conditions associated with climate change may revise this view. Efforts are underway to engineer plants to: (i) overcome the limitations to photosynthesis from today's [CO(2)] and (ii) benefit maximally from future, greater [CO(2)]. Targets range in scale from manipulating the function of a single enzyme (e.g. Rubisco) to adding metabolic pathways from bacteria as well as engineering the structural and functional components necessary for C(4) photosynthesis into C(3) leaves. Successfully improving plant performance will depend on combining the knowledge of the evolutionary context, cellular basis and physiological integration of plant responses to varying [CO(2)].
Figures
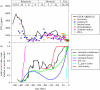
Figure 1.
Comparative time courses over most of the Phanerozoic of: (a) estimated atmospheric [CO2] predicted from a geochemical model of the carbon cycle (GEOCARBSULF, adapted from Berner [13]) and multiple proxies of [CO2] (stomatal indices and isotope analysis of liverworts, palaeosols, marine boron, phytoplankton and B/Ca, updated from the compilation of Royer [21]), with a dashed line at 1000 ppm indicating the atmospheric [CO2] above which photosynthesis is saturated in most modern plants [22]; (b) estimated maximum stomatal conductance (adapted from Franks & Beerling [23]), estimated vascular species richness (adapted from Knoll & Niklas [24]), stomatal density (redrawn from Royer et al. [20]), Devonian and Carboniferous leaf size (adapted from Osborne et al. [25]), C4 grass clade richness (adapted from Edwards et al. [9]) and angiosperm vein density (adapted from Brodribb & Feild [26]), all of which are expressed relative to the maximum value in the individual records of each parameter from the cited studies.
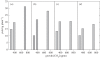
Figure 2.
Seed yield as a function of growth at [CO2] ranging from subambient (293 ppm) to ambient (385 ppm) and elevated [CO2] (715 ppm) for four different Spring wheat lines released in (a) 1903 (Marquis), (b) 1921 (Thatcher), (c) 1965 (Chris) and (d) 1996 (Oxen). Treatment means are adapted from Ziska et al. [60].
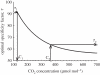
Figure 3.
Assuming a fixed number of Rubisco active sites per unit leaf area and the dependence of catalytic rate per active site  on specificity described for different photosynthetic organisms by Zhu et al. [116], the line shows, for any given atmospheric [CO2], the specificity (τ) that will give the highest light-saturated rate of leaf photosynthetic CO2 uptake (_A_sat). The average τ for terrestrial C3 crop plants (92.5) is indicated (_τ_1) together with the interpolated atmospheric [CO2] at which it would yield the maximum _A_sat (C1). Point _τ_2 is the specificity that would yield the highest _A_sat at the current [CO2] of the atmosphere (C2). At C2, decrease in τ from present average (_τ_1) to the optimum for current [CO2] (_τ_2) can increase light-saturated leaf photosynthetic carbon uptake by 12%. Reproduced with permission from Zhu et al. [116].
on specificity described for different photosynthetic organisms by Zhu et al. [116], the line shows, for any given atmospheric [CO2], the specificity (τ) that will give the highest light-saturated rate of leaf photosynthetic CO2 uptake (_A_sat). The average τ for terrestrial C3 crop plants (92.5) is indicated (_τ_1) together with the interpolated atmospheric [CO2] at which it would yield the maximum _A_sat (C1). Point _τ_2 is the specificity that would yield the highest _A_sat at the current [CO2] of the atmosphere (C2). At C2, decrease in τ from present average (_τ_1) to the optimum for current [CO2] (_τ_2) can increase light-saturated leaf photosynthetic carbon uptake by 12%. Reproduced with permission from Zhu et al. [116].
Similar articles
- One crop breeding cycle from starvation? How engineering crop photosynthesis for rising CO2 and temperature could be one important route to alleviation.
Kromdijk J, Long SP. Kromdijk J, et al. Proc Biol Sci. 2016 Mar 16;283(1826):20152578. doi: 10.1098/rspb.2015.2578. Proc Biol Sci. 2016. PMID: 26962136 Free PMC article. Review. - Improving photosynthesis through the enhancement of Rubisco carboxylation capacity.
Iñiguez C, Aguiló-Nicolau P, Galmés J. Iñiguez C, et al. Biochem Soc Trans. 2021 Nov 1;49(5):2007-2019. doi: 10.1042/BST20201056. Biochem Soc Trans. 2021. PMID: 34623388 Review. - Physiological and molecular alterations in plants exposed to high [CO2] under phosphorus stress.
Pandey R, Zinta G, AbdElgawad H, Ahmad A, Jain V, Janssens IA. Pandey R, et al. Biotechnol Adv. 2015 May-Aug;33(3-4):303-16. doi: 10.1016/j.biotechadv.2015.03.011. Epub 2015 Mar 20. Biotechnol Adv. 2015. PMID: 25797341 Review. - Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends.
Galmés J, Kapralov MV, Andralojc PJ, Conesa MÀ, Keys AJ, Parry MA, Flexas J. Galmés J, et al. Plant Cell Environ. 2014 Sep;37(9):1989-2001. doi: 10.1111/pce.12335. Epub 2014 May 11. Plant Cell Environ. 2014. PMID: 24689692 - Natural genetic variation in plant photosynthesis.
Flood PJ, Harbinson J, Aarts MG. Flood PJ, et al. Trends Plant Sci. 2011 Jun;16(6):327-35. doi: 10.1016/j.tplants.2011.02.005. Epub 2011 Mar 23. Trends Plant Sci. 2011. PMID: 21435936 Review.
Cited by
- Rapid evolution of Medicago polymorpha during invasion shifts interactions with the soybean looper.
Jack CN, Friesen ML. Jack CN, et al. Ecol Evol. 2019 Aug 16;9(18):10522-10533. doi: 10.1002/ece3.5572. eCollection 2019 Sep. Ecol Evol. 2019. PMID: 31632647 Free PMC article. - Photosynthetic variation and responsiveness to CO2 in a widespread riparian tree.
Dillon S, Quentin A, Ivković M, Furbank RT, Pinkard E. Dillon S, et al. PLoS One. 2018 Jan 2;13(1):e0189635. doi: 10.1371/journal.pone.0189635. eCollection 2018. PLoS One. 2018. PMID: 29293528 Free PMC article. - Carbon for nutrient exchange between Lycopodiella inundata and Mucoromycotina fine root endophytes is unresponsive to high atmospheric CO2.
Hoysted GA, Kowal J, Pressel S, Duckett JG, Bidartondo MI, Field KJ. Hoysted GA, et al. Mycorrhiza. 2021 Jul;31(4):431-440. doi: 10.1007/s00572-021-01033-6. Epub 2021 Apr 21. Mycorrhiza. 2021. PMID: 33884466 Free PMC article. - Plant-plant interactions mediate the plastic and genotypic response of Plantago asiatica to CO2: an experiment with plant populations from naturally high CO2 areas.
van Loon MP, Rietkerk M, Dekker SC, Hikosaka K, Ueda MU, Anten NP. van Loon MP, et al. Ann Bot. 2016 Jun;117(7):1197-207. doi: 10.1093/aob/mcw064. Epub 2016 Apr 27. Ann Bot. 2016. PMID: 27192707 Free PMC article. - Natural variation in stress response induced by low CO2 in Arabidopsis thaliana.
Wu C, Sun Y, Yang G, Li L, Sun W, Wang Z, Zhang H, Li Y. Wu C, et al. Open Life Sci. 2020 Dec 21;15(1):923-938. doi: 10.1515/biol-2020-0095. eCollection 2020. Open Life Sci. 2020. PMID: 33817279 Free PMC article.
References
- Ainsworth E. A., Rogers A., Leakey A. D. B. 2008. Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol. 147, 13–1910.1104/pp.108.117101 (doi:10.1104/pp.108.117101) - DOI - DOI - PMC - PubMed
- Beerling D. J. 2009. Coevolution of photosynthetic organisms and the environment. Geobiology 7, 97–9910.1111/j.1472-4669.2009.00196.x (doi:10.1111/j.1472-4669.2009.00196.x) - DOI - DOI - PubMed
- Ward J. K., Kelly J. K. 2004. Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecol. Lett. 7, 427–44010.1111/j.1461-0248.2004.00589.x (doi:10.1111/j.1461-0248.2004.00589.x) - DOI - DOI
- Ward J. K., Strain B. R. 1999. Elevated CO2 studies: past, present and future. Tree Physiol. 19, 211–22010.1093/treephys/19.4-5.211 (doi:10.1093/treephys/19.4-5.211) - DOI - DOI - PubMed
- Ackerly D. D., et al. 2000. The evolution of plant ecophysiological traits: recent advances and future directions. BioScience 50, 979–99510.1641/0006-3568(2000)050[0979:TEOPET]2.0.CO;2 (doi:10.1641/0006-3568(2000)050[0979:TEOPET]2.0.CO;2) - DOI - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous