Dynamin, a membrane-remodelling GTPase - PubMed (original) (raw)
Review
Dynamin, a membrane-remodelling GTPase
Shawn M Ferguson et al. Nat Rev Mol Cell Biol. 2012.
Abstract
Dynamin, the founding member of a family of dynamin-like proteins (DLPs) implicated in membrane remodelling, has a critical role in endocytic membrane fission events. The use of complementary approaches, including live-cell imaging, cell-free studies, X-ray crystallography and genetic studies in mice, has greatly advanced our understanding of the mechanisms by which dynamin acts, its essential roles in cell physiology and the specific function of different dynamin isoforms. In addition, several connections between dynamin and human disease have also emerged, highlighting specific contributions of this GTPase to the physiology of different tissues.
Figures
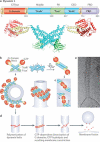
Figure 1. Sites of action of dynamin and dynamin related proteins (DLPs) in a mammalian cell
(a) Dynamin is localized at sites of endocytosis. It is also found at actin meshworks nucleated by the Arp2/3 complex such as membrane ruffles, podosomes and invadopodia, and at actin pedestals induced by pathogenic bacteria. Other DLPs, including atlastin, Drp1, OPA1 and mitofusin, localize to sites of intracellular membrane fission and fusion in the endoplasmic reticulum and mitochondria. The location of dynamin and DLPs is shown in red while F-actin is coloured green. The endosymbiotic origin of mitochondria (i.e. their formation by the endocytosis of an ancient prokaryote) may explain the role of a DLP in the fission of their outer mitochondrial membrane
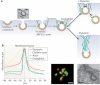
Figure 2. Structure of dynamin and putative mechanism of dynamin-mediated membrane fission
(a) Top: Linear representation of the domain organization of dynamin based on its 3D structure as revealed by crystallographic studies (numbers indicate amino acid position within the primary sequence of human dynamin 1, xa splice variant). Regions that belong to the same folded module are shown in the same color. Bottom: crystal structure of a dynamin dimer (color coded to match the linear representation). Molecular graphics were created with Pymol, PDB code 3SNH ). (b) Schematic representation of dynamin dimers and of helical dynamin polymers around a tubular template in two different orientations (90 degrees rotation). The colour-coding of the domains matches the colors of panel (a). The approximate location of the bound nucleotide is highlighted in yellow. Dynamin polymerization occurs as a result of interactions between the stalks of dynamin monomers (interface 2) and between stalk dimers (interfaces 1 and 3). The GTP-dependent dimerization of G domains between adjacent rungs of the dynamin helix (highlighted in yellow stars, longitudinal view of the helix), is thought to promote assembly-stimulated GTPase activity, resulting in membrane constriction and ultimately fission. (d) Proposed GTP hydrolysis-dependent lever-like movement of dynamin's neck (BSE) relative to the G domain. (e) Schematic view of the key steps leading to dynamin-mediated membrane fission. (e) Cryo-EM image showing a helical polymer of purified dynamin that has driven the formation of a tubule from a liposome. Image kindly provided by Adam Frost and Vincenz Unger (University of Utah and Northwestern University respectively).
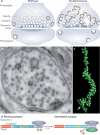
Figure 3. Dynamin and clathrin-mediated endocytosis
(a) Putative sequence of action of actin, BAR proteins and dynamin at clathrin coated endocytic pits as revealed by studies of cells that lack dynamin (dynamin 1,2 double KO fibroblasts) . Lack of dynamin results in an arrest of the endocytic reaction at the stage of deeply invaginated pits and in the actin-dependent elongation of their BAR protein coated tubular necks (EM micrograph at lower right, reproduced with permission from ). When actin is depolymerized (latrunculin B treatment), clathrin coated buds collapse to omega shaped pits with short wide necks (upper left EM micrograph, reproduced with permission from ). The fluorescence micrograph shows tubulated endocytic clathrin coated pits in dynamin 1,2 double KO fibroblasts as revealed by live cell imaging of the BAR protein endophilin 2-GFP (tubular neck) and of RFP-clathrin light chain (reproduced with permission from ) (b) Schematic timeline showing the accumulation of endocytic proteins at endocytic pits. The zero time represents the fission reaction, as determined by the loss of accessibility of the bud lumen to the extracellular medium (sketched based on data from ). Note that the role of actin may be non-essential under some conditions , .
Figure 4. Dynamin participates in synaptic vesicle recycling at neuronal synapses
(a) From electron microscopy analysis, wild type synapses (left) are characterized by an abundance of synaptic vesicles and very few clathrin-coated pits or clathrin-coated vesicles, consistent with the transient nature of clathrin-coated structures even during intense synaptic activity. At synapses that lack the great majority of their normal dynamin content (for example, from dynamin 1 knockout neurons or even more strikingly from dynamin 1,3 double KO neurons) (right) there is a depletion of synaptic vesicles and a striking accumulation of uniformly sized clathrin coated pits which bud from long invaginations of the plasma membrane , , . A small increase in the abundance of clathrin coated pits is also observed post-synaptically, where pits are heterogeneous in size. (b) Representative electron micrograph of a presynaptic terminal from a dynamin 1 knockout neuron. Presynaptic vesicle clusters at two synaptic junctions are very small (regions defined by dashed lines) while clathrin-coated vesicular structures are highly abundant and occupy the bulk of the terminal. As shown by electron tomography (see field c), all such structures are coated pits, although in this thin section (~60nm) their tubular stalks can only seen in few cases and their connection with the plasma membrane cannot be appreciated , . (c) Partial electron tomography reconstruction from a dynamin 1,3 double KO nerve terminals showing a long tubular plasma membrane invagination studded with numerous individual clathrin coated pits. The arrow indicates the opening of this tubular structure at the peripheral presynaptic plasma membrane. (d) In resting synapses, the PRD region of dynamin 1 is phosphorylated, preventing association with syndapin. Upon stimulation and increased cytosolic [Ca2+], the protein phosphatase calcineurin, which directly binds a dynamin 1 splice variant (xb), dephosphorylates dynamin 1, allowing its interaction with syndapin. This regulated interaction is thought to promote synaptic vesicle endocytosis at times of elevated neuronal activity.
Similar articles
- Dynamin: functional design of a membrane fission catalyst.
Schmid SL, Frolov VA. Schmid SL, et al. Annu Rev Cell Dev Biol. 2011;27:79-105. doi: 10.1146/annurev-cellbio-100109-104016. Epub 2011 May 18. Annu Rev Cell Dev Biol. 2011. PMID: 21599493 Review. - Dynamin GTPase, a force-generating molecular switch.
Warnock DE, Schmid SL. Warnock DE, et al. Bioessays. 1996 Nov;18(11):885-93. doi: 10.1002/bies.950181107. Bioessays. 1996. PMID: 8939066 Review. - Dynamin: characteristics, mechanism of action and function.
Wiejak J, Wyroba E. Wiejak J, et al. Cell Mol Biol Lett. 2002;7(4):1073-80. Cell Mol Biol Lett. 2002. PMID: 12511974 Review. - Dynamin-catalyzed membrane fission requires coordinated GTP hydrolysis.
Liu YW, Mattila JP, Schmid SL. Liu YW, et al. PLoS One. 2013;8(1):e55691. doi: 10.1371/journal.pone.0055691. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383266 Free PMC article. - A corkscrew model for dynamin constriction.
Mears JA, Ray P, Hinshaw JE. Mears JA, et al. Structure. 2007 Oct;15(10):1190-202. doi: 10.1016/j.str.2007.08.012. Structure. 2007. PMID: 17937909 Free PMC article.
Cited by
- Minireview: Spatial Programming of G Protein-Coupled Receptor Activity: Decoding Signaling in Health and Disease.
West C, Hanyaloglu AC. West C, et al. Mol Endocrinol. 2015 Aug;29(8):1095-106. doi: 10.1210/ME.2015-1065. Epub 2015 Jun 29. Mol Endocrinol. 2015. PMID: 26121235 Free PMC article. Review. - Endocytosis of the thrombopoietin receptor Mpl regulates megakaryocyte and erythroid maturation in mice.
Eaton N, Boyd EK, Biswas R, Lee-Sundlov MM, Dlugi TA, Ramsey HE, Zheng S, Burns RT, Sola-Visner MC, Hoffmeister KM, Falet H. Eaton N, et al. Front Oncol. 2022 Aug 30;12:959806. doi: 10.3389/fonc.2022.959806. eCollection 2022. Front Oncol. 2022. PMID: 36110936 Free PMC article. - Integration of the Rac1- and actin-binding properties of Coronin-1C.
Tilley FC, Williamson RC, Race PR, Rendall TC, Bass MD. Tilley FC, et al. Small GTPases. 2015;6(1):36-42. doi: 10.4161/21541248.2014.992259. Small GTPases. 2015. PMID: 25862165 Free PMC article. - Entry of Classical Swine Fever Virus into PK-15 Cells via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway That Requires Rab5 and Rab7.
Shi BJ, Liu CC, Zhou J, Wang SQ, Gao ZC, Zhang XM, Zhou B, Chen PY. Shi BJ, et al. J Virol. 2016 Sep 29;90(20):9194-208. doi: 10.1128/JVI.00688-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489278 Free PMC article. - Free energy calculations for membrane morphological transformations and insights to physical biology and oncology.
Parihar K, Ko SH, Bradley R, Taylor P, Ramakrishnan N, Baumgart T, Guo W, Weaver VM, Janmey PA, Radhakrishnan R. Parihar K, et al. Methods Enzymol. 2024;701:359-386. doi: 10.1016/bs.mie.2024.03.028. Epub 2024 Apr 14. Methods Enzymol. 2024. PMID: 39025576 Free PMC article.
References
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature reviews. Molecular cell biology. 2011;12:517–33. - PubMed
- Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annual review of biochemistry. 2010;79:803–33. - PubMed
- Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–73. - PubMed
- Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Current opinion in cell biology. 2010;22:519–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- P30 DA018343/DA/NIDA NIH HHS/United States
- DK45735/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- DA018343/DA/NIDA NIH HHS/United States
- R37NS036251/NS/NINDS NIH HHS/United States
- R37 NS036251/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources