Bevacizumab added to neoadjuvant chemotherapy for breast cancer - PubMed (original) (raw)
Randomized Controlled Trial
. 2012 Jan 26;366(4):310-20.
doi: 10.1056/NEJMoa1111097.
Gong Tang, Priya Rastogi, Charles E Geyer Jr, André Robidoux, James N Atkins, Luis Baez-Diaz, Adam M Brufsky, Rita S Mehta, Louis Fehrenbacher, James A Young, Francis M Senecal, Rakesh Gaur, Richard G Margolese, Paul T Adams, Howard M Gross, Joseph P Costantino, Sandra M Swain, Eleftherios P Mamounas, Norman Wolmark
Affiliations
- PMID: 22276821
- PMCID: PMC3401076
- DOI: 10.1056/NEJMoa1111097
Randomized Controlled Trial
Bevacizumab added to neoadjuvant chemotherapy for breast cancer
Harry D Bear et al. N Engl J Med. 2012.
Abstract
Background: Bevacizumab and the antimetabolites capecitabine and gemcitabine have been shown to improve outcomes when added to taxanes in patients with metastatic breast cancer. The primary aims of this trial were to determine whether the addition of capecitabine or gemcitabine to neoadjuvant chemotherapy with docetaxel, followed by doxorubicin plus cyclophosphamide, would increase the rates of pathological complete response in the breast in women with operable, human epidermal growth factor receptor 2 (HER2)-negative breast cancer and whether adding bevacizumab to these chemotherapy regimens would increase the rates of pathological complete response.
Methods: We randomly assigned 1206 patients to receive neoadjuvant therapy consisting of docetaxel (100 mg per square meter of body-surface area on day 1), docetaxel (75 mg per square meter on day 1) plus capecitabine (825 mg per square meter twice a day on days 1 to 14), or docetaxel (75 mg per square meter on day 1) plus gemcitabine (1000 mg per square meter on days 1 and 8) for four cycles, with all regimens followed by treatment with doxorubicin-cyclophosphamide for four cycles. Patients were also randomly assigned to receive or not to receive bevacizumab (15 mg per kilogram of body weight) for the first six cycles of chemotherapy.
Results: The addition of capecitabine or gemcitabine to docetaxel therapy, as compared with docetaxel therapy alone, did not significantly increase the rate of pathological complete response (29.7% and 31.8%, respectively, vs. 32.7%; P=0.69). Both capecitabine and gemcitabine were associated with increased toxic effects--specifically, the hand-foot syndrome, mucositis, and neutropenia. The addition of bevacizumab significantly increased the rate of pathological complete response (28.2% without bevacizumab vs. 34.5% with bevacizumab, P=0.02). The effect of bevacizumab on the rate of pathological complete response was not the same in the hormone-receptor-positive and hormone-receptor-negative subgroups. The addition of bevacizumab increased the rates of hypertension, left ventricular systolic dysfunction, the hand-foot syndrome, and mucositis.
Conclusions: The addition of bevacizumab to neoadjuvant chemotherapy significantly increased the rate of pathological complete response, which was the primary end point of this study. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00408408.).
Figures
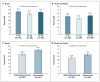
Figure 1. Pathological Complete Response (pCR)
The percentages of patients with pCR in the breast (Panel A) and in the breast and nodes (Panel B) are shown according to chemotherapy regimen (docetaxel followed by doxorubicin–cyclophosphamide [T–AC], docetaxel and capecitabine followed by doxorubicin–cyclophosphamide [TX–AC], or docetaxel and gemcitabine followed by doxorubicin–cyclophosphamide [TG–AC]). In addition, the percentages of patients with pCR in the breast (Panel C) and in the breast and nodes (Panel D) are shown according to receipt or no receipt of bevacizumab.
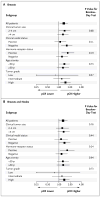
Figure 2. Subgroup Analyses of the Effect of Bevacizumab on Pathological Complete Response (pCR)
The effect of bevacizumab with respect to pCR in the breast (Panel A) and in the breast and nodes (Panel B) is shown across various subgroups of eligible patients. P values were calculated from tests for the homogeneity of the effects of bevacizumab across patient subgroups.
Comment in
- Fighting fire with fire: rekindling the bevacizumab debate.
Montero AJ, Vogel C. Montero AJ, et al. N Engl J Med. 2012 Jan 26;366(4):374-5. doi: 10.1056/NEJMe1113368. N Engl J Med. 2012. PMID: 22276827 No abstract available. - Bevacizumab in neoadjuvant treatment for breast cancer.
van der Veldt AA, Smit EF. van der Veldt AA, et al. N Engl J Med. 2012 Apr 26;366(17):1637; author reply 1638-40. doi: 10.1056/NEJMc1202229. N Engl J Med. 2012. PMID: 22533581 No abstract available. - Bevacizumab in neoadjuvant treatment for breast cancer.
Somlo G. Somlo G. N Engl J Med. 2012 Apr 26;366(17):1637-8; author reply 1638-40. doi: 10.1056/NEJMc1202229. N Engl J Med. 2012. PMID: 22533582 No abstract available.
Similar articles
- Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): secondary outcomes of a phase 3, randomised controlled trial.
Bear HD, Tang G, Rastogi P, Geyer CE Jr, Liu Q, Robidoux A, Baez-Diaz L, Brufsky AM, Mehta RS, Fehrenbacher L, Young JA, Senecal FM, Gaur R, Margolese RG, Adams PT, Gross HM, Costantino JP, Paik S, Swain SM, Mamounas EP, Wolmark N. Bear HD, et al. Lancet Oncol. 2015 Sep;16(9):1037-1048. doi: 10.1016/S1470-2045(15)00041-8. Epub 2015 Aug 10. Lancet Oncol. 2015. PMID: 26272770 Free PMC article. Clinical Trial. - Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer.
von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, Solbach C, Gerber B, Jackisch C, Kunz G, Blohmer JU, Huober J, Hauschild M, Fehm T, Müller BM, Denkert C, Loibl S, Nekljudova V, Untch M; German Breast Group; Arbeitsgemeinschaft Gynäkologische Onkologie–Breast Study Groups. von Minckwitz G, et al. N Engl J Med. 2012 Jan 26;366(4):299-309. doi: 10.1056/NEJMoa1111065. N Engl J Med. 2012. PMID: 22276820 Clinical Trial. - Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study.
von Minckwitz G, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B, Hanusch C, Kühn T, du Bois A, Blohmer JU, Thomssen C, Dan Costa S, Jackisch C, Kaufmann M, Mehta K, Untch M. von Minckwitz G, et al. J Clin Oncol. 2010 Apr 20;28(12):2015-23. doi: 10.1200/JCO.2009.23.8303. Epub 2010 Mar 22. J Clin Oncol. 2010. PMID: 20308671 Clinical Trial. - Bevacizumab in combination with a taxane for the first-line treatment of HER2-negative metastatic breast cancer.
Rodgers M, Soares M, Epstein D, Yang H, Fox D, Eastwood A. Rodgers M, et al. Health Technol Assess. 2011 May;15 Suppl 1:1-12. doi: 10.3310/hta15suppl1/01. Health Technol Assess. 2011. PMID: 21609648 Review. - Sorafenib in locally advanced or metastatic breast cancer.
Gradishar WJ. Gradishar WJ. Expert Opin Investig Drugs. 2012 Aug;21(8):1177-91. doi: 10.1517/13543784.2012.689824. Epub 2012 May 23. Expert Opin Investig Drugs. 2012. PMID: 22616580 Review.
Cited by
- Protein Signature Predicts Response to Neoadjuvant Treatment With Chemotherapy and Bevacizumab in HER2-Negative Breast Cancers.
Haugen MH, Lingjærde OC, Hedenfalk I, Garred Ø, Borgen E, Loman N, Hatschek T, Børresen-Dale AL, Naume B, Mills GB, Mælandsmo GM, Engebraaten O. Haugen MH, et al. JCO Precis Oncol. 2021 Jan 28;5:PO.20.00086. doi: 10.1200/PO.20.00086. eCollection 2021. JCO Precis Oncol. 2021. PMID: 34036235 Free PMC article. Clinical Trial. - Current approaches for neoadjuvant chemotherapy in breast cancer.
Connolly RM, Stearns V. Connolly RM, et al. Eur J Pharmacol. 2013 Oct 5;717(1-3):58-66. doi: 10.1016/j.ejphar.2013.02.057. Epub 2013 Mar 29. Eur J Pharmacol. 2013. PMID: 23545358 Free PMC article. Review. - Dynamic contrast-enhanced MR imaging in a phase Ⅱ study on neoadjuvant chemotherapy combining Rh-endostatin with docetaxel and epirubicin for locally advanced breast cancer.
Jia Q, Xu J, Jiang W, Zheng M, Wei M, Chen J, Wang L, Huan Y. Jia Q, et al. Int J Med Sci. 2013;10(2):110-8. doi: 10.7150/ijms.5123. Epub 2012 Dec 28. Int J Med Sci. 2013. PMID: 23329881 Free PMC article. Clinical Trial. - Molecular Biology in the Breast Clinics-Current status and future perspectives.
Parmar V, Nair NS, Thakkar P, Chitkara G. Parmar V, et al. Indian J Surg Oncol. 2021 Apr;12(Suppl 1):7-20. doi: 10.1007/s13193-019-00954-1. Epub 2019 Aug 10. Indian J Surg Oncol. 2021. PMID: 33994723 Free PMC article. Review.
References
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93. - PubMed
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85. - PubMed
- Gianni L, Baselga J, Eiermann W, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol. 2009;27:2474–81. - PubMed
- Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–702. Erratum, J Clin Oncol 2005;23:4808. - PubMed
- Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to pre-operative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10-CA-37377/CA/NCI NIH HHS/United States
- U10 CA069974-16/CA/NCI NIH HHS/United States
- U10 CA044066-25/CA/NCI NIH HHS/United States
- U10 CA012027/CA/NCI NIH HHS/United States
- U10 CA069651/CA/NCI NIH HHS/United States
- U10 CA069651-18/CA/NCI NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
- U10 CA044066/CA/NCI NIH HHS/United States
- U10 CA037377-26/CA/NCI NIH HHS/United States
- U10 CA180844/CA/NCI NIH HHS/United States
- U10 CA037377/CA/NCI NIH HHS/United States
- U10-CA-69651/CA/NCI NIH HHS/United States
- U10-CA-44066/CA/NCI NIH HHS/United States
- U10-CA-69974/CA/NCI NIH HHS/United States
- U10-CA-12027/CA/NCI NIH HHS/United States
- U10 CA069974/CA/NCI NIH HHS/United States
- U10 CA012027-35/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous