Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles - PubMed (original) (raw)
Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles
Gina M Donato et al. FEBS Lett. 2012.
Abstract
Bordetella pertussis adenylate cyclase toxin (ACT) intoxicates cells by producing intracellular cAMP. B. pertussis outer membrane vesicles (OMV) contain ACT on their surface (OMV-ACT), but the properties of OMV-ACT were previously unknown. We found that B. pertussis in the lung from a fatal pertussis case contains OMV, suggesting an involvement in pathogenesis. OMV-ACT and ACT intoxicate cells with and without the toxin's receptor CD11b/CD18. Intoxication by ACT is blocked by antitoxin and anti-CD11b antibodies, but not by cytochalasin-D; in contrast, OMV-ACT is unaffected by either antibody and blocked by cytochalasin-D. Thus OMV-ACT can deliver ACT by processes distinct from those of ACT alone.
Copyright © 2012 Federation of European Biochemical Societies. All rights reserved.
Figures
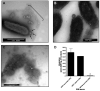
Figure 1. B.pertussis organisms and OMV in human pathology specimens
Electron microscopic images of outer membrane vesicles associated with B.pertussis are from lung tissue obtained at autopsy from a child with fatal B. pertussis infection. Arrows indicate OMV. Scale bar=0.1 μm; magnification=30,000X.
Figure 2. Transmission EM images of B.pertussis and OMV
A. B.pertussis GMT1 and associated OMV were negatively stained with 2% NanoVan. Arrows indicate OMV attached to the bacterial surface; bracket represents OMV released into the culture medium; scale bar=1 μm; magnification=15,000X. B. Ultrathin section of embedded bacteria and attached OMV stained with uranyl acetate and lead citrate. Scale bar=0.2 μm; magnification=40,000X. C. OMV prepared and purified from GMT1 by the method of Hozbor et al. [19] were negatively stained with 2% PTA pH 7.0. Scale bar=0.5 μm; magnification=30,000X. D. J774.A1 cells were intoxicated comparably by enriched and native OMV from GMT1. The comparison of intoxication was based on equalized AC enzymatic activities. Enriched OMV from the ACT-negative strain, BP348, did not cause intoxication. * p<0.0001 compared to GMT1 enriched OMV.
Figure 3. Distribution of periplasmic and cytoplasmic markers in B.pertussis and OMV
A. GMT1(pTH22), a B. pertussis strain expressing the periplasmic enzyme marker, AP, was fractionated and the periplasmic and cytoplasmic fractions assayed for AP activity. Data represent the blanked mean ± S.E.M. from 4 independent experiments. B. Enriched OMV from GMT1(pTH22) were lysed and assayed for AP enzyme activity. AP activity was detected in the OMV lysate and luminal fraction, but not the membrane fraction. Representative graph from 3 independent experiments. C. Enriched GMT1 OMV were lysed and assayed for MDH via the oxidation of β-NADH, which is reflected as a decrease in OD340. MDH activity of the B pertussis lysate is shown in inset. Representative graph from 3 independent experiments.
Figure 4. Trypsin-sensitivity assay
OMV-ACT and purified ACT were incubated with or without 40 μg/ml trypsin for 5 min at 37°C and then, 80 μg/ml trypsin inhibitor was added. As a control, samples were incubated with trypsin inhibitor prior to trypsin treatment. These samples were assayed for (A) adenylate cyclase enzymatic activity and (B) intoxication. Data reported as a percentage of the untreated controls, which were set at 100%. At equivalent ACT enzymatic activity, intoxication for purified ACT was 9215 ± 1241 pmol cAMP/mg J774A.1 protein and for OMV-ACT it was 9896 ± 270 pmol cAMP/mg J774A.1 protein. All data represent the mean ± S.E.M. with background subtracted of duplicate samples from at least 2 independent experiments. * p<0.007 compared to untreated controls.
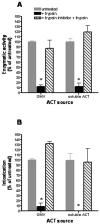
Figure 5. Effect of antibodies and cytochalasin-D on intoxication by ACT and OMV-ACT
A. OMV-ACT and purified ACT were incubated with 20 μg/ml anti-ACT (3D1) or control antibody (mouse isotype IgG1) for 10 min at 4°C and then added directly to J774A.1 cells. Alternatively, J774A.1 macrophages were pre-treated with 10 μg/ml anti-CD11b or control antibody (rat isotype IgG2b) at 4°C for 1 hr before purified ACT or OMV-ACT was added. Cells were incubated at 37°C for 1 hr and then cAMP measured. Data reported as a percentage of the untreated controls, which were set at 100%. At equivalent ACT enzymatic activity, intoxication for ACT was 10280 ± 1786 pmol cAMP/mg J774A.1 protein and for OMV-ACT it was 5117 ± 442 pmol cAMP/mg J774A.1 protein. All data represent the mean ± S.E.M. with background subtracted of duplicate samples from at least 2 independent experiments. * p<0.0018 compared to untreated controls. B. J774.A1 macrophages were incubated with or without 10 μg/ml cytochalasin-D for 1 hr at 37°C before OMV-ACT or purified ACT was added and incubated an additional hour at 37°C. * p=0.0009 compared to untreated control. C. J774.A1 and CHO cells were each treated with cytochalasin-D as described in (5B), equal amounts of OMV-ACT were added and incubated an additional hour at 37°C. Buffer control was 121 ± 23 pmol cAMP/mg cell protein; DMSO-treated control cells was 37 ± 6 pmol cAMP/mg cell protein. All data represent the mean ± S.E.M. of duplicate samples from at least 2 independent experiments. * p<0.0045 compared to untreated controls.
Similar articles
- Physiopathological roles of spontaneously released outer membrane vesicles of Bordetella pertussis.
Gasperini G, Arato V, Pizza M, Aricò B, Leuzzi R. Gasperini G, et al. Future Microbiol. 2017 Nov;12:1247-1259. doi: 10.2217/fmb-2017-0064. Epub 2017 Oct 5. Future Microbiol. 2017. PMID: 28980823 - Bordetella pertussis adenylate cyclase toxin: intoxication of host cells by bacterial invasion.
Mouallem M, Farfel Z, Hanski E. Mouallem M, et al. Infect Immun. 1990 Nov;58(11):3759-64. doi: 10.1128/iai.58.11.3759-3764.1990. Infect Immun. 1990. PMID: 2172167 Free PMC article. - Selective Enhancement of the Cell-Permeabilizing Activity of Adenylate Cyclase Toxin Does Not Increase Virulence of Bordetella pertussis.
Holubova J, Juhasz A, Masin J, Stanek O, Jurnecka D, Osickova A, Sebo P, Osicka R. Holubova J, et al. Int J Mol Sci. 2021 Oct 28;22(21):11655. doi: 10.3390/ijms222111655. Int J Mol Sci. 2021. PMID: 34769101 Free PMC article. - Pertussis toxin and adenylate cyclase toxin: key virulence factors of Bordetella pertussis and cell biology tools.
Carbonetti NH. Carbonetti NH. Future Microbiol. 2010 Mar;5(3):455-69. doi: 10.2217/fmb.09.133. Future Microbiol. 2010. PMID: 20210554 Free PMC article. Review. - Molecular aspects of Bordetella pertussis pathogenesis.
Locht C. Locht C. Int Microbiol. 1999 Sep;2(3):137-44. Int Microbiol. 1999. PMID: 10943406 Review.
Cited by
- Cyclic AMP-Elevating Capacity of Adenylate Cyclase Toxin-Hemolysin Is Sufficient for Lung Infection but Not for Full Virulence of Bordetella pertussis.
Skopova K, Tomalova B, Kanchev I, Rossmann P, Svedova M, Adkins I, Bibova I, Tomala J, Masin J, Guiso N, Osicka R, Sedlacek R, Kovar M, Sebo P. Skopova K, et al. Infect Immun. 2017 May 23;85(6):e00937-16. doi: 10.1128/IAI.00937-16. Print 2017 Jun. Infect Immun. 2017. PMID: 28396322 Free PMC article. - Bordetella pertussis Adenylate Cyclase Toxin Disrupts Functional Integrity of Bronchial Epithelial Layers.
Hasan S, Kulkarni NN, Asbjarnarson A, Linhartova I, Osicka R, Sebo P, Gudmundsson GH. Hasan S, et al. Infect Immun. 2018 Feb 20;86(3):e00445-17. doi: 10.1128/IAI.00445-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29203545 Free PMC article. - Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity.
Jiang Y, Kong Q, Roland KL, Curtiss R 3rd. Jiang Y, et al. Int J Med Microbiol. 2014 May;304(3-4):431-43. doi: 10.1016/j.ijmm.2014.02.006. Epub 2014 Feb 19. Int J Med Microbiol. 2014. PMID: 24631214 Free PMC article. - Outer membrane vesicles as platform vaccine technology.
van der Pol L, Stork M, van der Ley P. van der Pol L, et al. Biotechnol J. 2015 Sep;10(11):1689-706. doi: 10.1002/biot.201400395. Biotechnol J. 2015. PMID: 26912077 Free PMC article. Review. - Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields.
Mobarak H, Javid F, Narmi MT, Mardi N, Sadeghsoltani F, Khanicheragh P, Narimani S, Mahdipour M, Sokullu E, Valioglu F, Rahbarghazi R. Mobarak H, et al. Cell Commun Signal. 2024 Jan 30;22(1):80. doi: 10.1186/s12964-023-01414-8. Cell Commun Signal. 2024. PMID: 38291458 Free PMC article. Review.
References
- Pertussis Epidemiology Surveillance and Reporting. CDC. 2011 ( www.cdc.gov/pertussis/surv-reporting.html)
Publication types
MeSH terms
Substances
Grants and funding
- AI18000/AI/NIAID NIH HHS/United States
- K08 AI081900/AI/NIAID NIH HHS/United States
- T32 AI007046/AI/NIAID NIH HHS/United States
- R01 AI018000-29/AI/NIAID NIH HHS/United States
- R01 AI018000/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials