High prevalence of autoantibodies to hLAMP-2 in anti-neutrophil cytoplasmic antibody-associated vasculitis - PubMed (original) (raw)
Comparative Study
doi: 10.1681/ASN.2011090920. Epub 2012 Feb 9.
Henko Tadema, Eoin F McKinney, Alexandra Benharkou, Ricarda Brandes, Andrea Peschel, Virginie Hubert, Tjerk Feenstra, Gürkan Sengölge, Coen Stegeman, Peter Heeringa, Paul A Lyons, Kenneth G C Smith, Cees Kallenberg, Andrew J Rees
Affiliations
- PMID: 22323643
- PMCID: PMC3294304
- DOI: 10.1681/ASN.2011090920
Comparative Study
High prevalence of autoantibodies to hLAMP-2 in anti-neutrophil cytoplasmic antibody-associated vasculitis
Renate Kain et al. J Am Soc Nephrol. 2012 Mar.
Abstract
The involvement of autoantibodies to human lysosome-associated membrane protein-2 (hLAMP-2) in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis is controversial because of the absence of confirmatory data subsequent to the initial reports of their high prevalence in this disease. We characterized three assays for anti-hLAMP-2 antibodies: ELISA and Western blotting assays using unglycosylated recombinant hLAMP-2 expressed in Escherichia coli, and an indirect immunofluorescence assay using stably transfected ldlD cells that expressed glycosylated full-length hLAMP-2 on the plasma membrane. The assays detected autoantibodies to hLAMP-2 in human sera reproducibly and with comparable sensitivity and the assays gave the same results in 80.5% of the test panel of 40 selected positive and negative sera. In untreated patients at presentation, the frequencies of autoantibodies to LAMP-2 were 89%, 91%, and 80%, respectively, among three groups of patients with ANCA-associated vasculitis from Vienna, Austria (n=19); Groningen, the Netherlands (n=50) and Cambridge, United Kingdom (n=53). Prevalence of LAMP-2 autoantibodies was similar in both those with myeloperoxidase-ANCA and proteinase 3-ANCA. Furthermore, we detected LAMP-2 autoantibodies in two ANCA-negative patients. LAMP-2 autoantibodies rapidly became undetectable after the initiation of immunosuppressive treatment and frequently became detectable again during clinical relapse. We conclude that when robust assays are used, circulating autoantibodies to hLAMP-2 can be detected in most European patients with ANCA-associated vasculitis. Large-scale prospective studies are now needed to determine whether they are pathogenic or merely an epiphenomenon.
Figures
Figure 1.
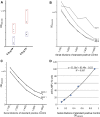
cDNA constructs, generation, and quality control of recombinant hLAMP-2. (A) Representation of cDNA encoding hLAMP-2A with the 28 amino acid leader peptide (LP), 347 amino acid extracellular domain, 24 amino acid transmembrane domain (TM), and 11 amino acid cytoplasmic domain (Cytopl). The two extracellular domain constructs were utilized to express soluble hLAMP-2 in E. coli (hLAMP-2/GST) and mammalian cells (hLAMP-2sol). Both contain the leader peptide but not the transmembrane domain or cytoplasmic tail. hLAMP-2sol expressed in mammalian cells results in an appropriately glycosylated soluble protein exported into the culture supernatant via the default secretory pathway in mammalian cells. The hLAMP-2 cytoplasmic tail contains the signal that directs its retrieval from the plasma membrane to lysosomes. The critical tyrosine was mutated to a histidine in hLAMP-2H (Y/H), which targets it to the plasma membrane when expressed in ldlD cells. (B) Purified hLAMP-2/GST runs as a single 65-kD band on SDS-PAGE and silver stain. Fractions of high purity were pooled and identity of hLAMP-2 was confirmed with an antibody reactive with hLAMP-2 only. (C) Purified hLAMP-2/GST (10 μg/ml) was separated by SDS-PAGE and transferred onto PVDF before probing with specific antibodies to hLAMP-2 (932b), which bound exclusively to the fusion protein. Antibody to GST (anti-GST) recognized both fusion protein and free GST. A human serum (1:100 dilution) containing anti-hLAMP-2 antibodies (Pat. 6) also bound the fusion protein, whereas serum from a healthy control (Healthy Co 50) did not. Serum from a patient with renal disease (Dis. Co 8) contained antibodies to GST. Secondary antibodies alone were negative (anti-human, anti-rabbit). (D) Sensitivity of the Western blot was assessed from doubling dilutions (1:50 to 1:400) of the standard positive control (Stand pos Co) used for all assays. This also confirmed the optimal binding/background ratio was 1:100.
Figure 2.
Development of anti-hLAMP-2 ELISA. (A) Comparison of ELISA plates coated with 0.5 and 5.0 µg/ml of recombinant hLAMP-2/GST fusion protein (FP). Plates coated with 5.0 µg/ml gave much better separation between positive and negative sera. (B) ELISA plates coated with hLAMP-2/GST FP were stored at 4°C and tested after different time points. Serial dilutions of a standard positive control serum gave similar results using plates stored for 1–33 days, but there was a considerable increase in background binding after 41 days. (C) Comparison of ELISA plates coated with two different batches of hLAMP-2/GST FP demonstrates comparable binding of serial dilutions of a standard positive control serum. (D) The standard curve derived from the results of assaying serial dilutions of the standard control serum was used to calculate anti-hLAMP-2 antibody concentrations with 100 U equating to OD of the 1:100 dilution of the positive control.
Figure 3.
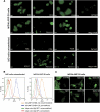
Indirect immunofluorescence on ldlD cells stably expressing hLAMP-2 on the cell surface (ldlD/hLAMP-2H). (A) ldlD cells were transfected with full-length hLAMP-2 cDNA with a tyrosine to histidine mutation in the cytoplasmic lysosomal retrieval signal. Stable transfectants were sorted by flow cytometry for uniform expression of the transgene and stained with monoclonal (CD3) and polyclonal (932B) antibodies to hLAMP-2 generated by M. Fukuda as well as commercially available monoclonal (H4B4) and polyclonal anti-LAMP-2 antibodies (SCN17 and Abnova). All of the antibodies bound to hLAMP-2 on the surface of nonpermeabilized transfected cells (np). The commercial anti-hLAMP-2 cross-reacted with hamster LAMP-2 in transfected and untransfected cells especially after permeabilization (p). CD3 and 932B were the only antibodies that did not cross-react with hamster LAMP-2. A polyclonal antibody to rat LAMP-2 (Zymed Laboratories) and secondary antibody alone fail to detect either human or hamster LAMP-2. (B) FACS analysis of ldlD/hLAMP-2H cells and control ldlD cells stained with H4B4, a mAb to hLAMP-2 that cross-reacts with hamster LAMP-2. Surface expression of hLAMP-2 is detected only in transfected cells, whereas intracellular staining of native hamster LAMP-2 is apparent after permeabilization with saponin. (C) The sensitivity of the IIF assay for antibodies to hLAMP-2 assessed from doubling dilutions (1:50 to 1:800) of the standard positive control (Stand Co) compared with binding of monoclonal anti-hLAMP-2 antibody (H4B4; pos Co) and negative control serum (neg Co). IgG binding to ldlD/hLAMP-2H cells decreases progressively in titers >1:100 and becomes negative at 1:400, which is 10-fold higher than the standard assay dilution of 1:40.
Figure 4.
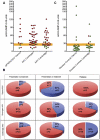
Evaluation of assays for antibodies to LAMP-2. (A) ELISA results from a panel of sera from 78 healthy controls were used to derive the mean ± SD for this group and the 95% confidence limit of the upper limit of normal for the assay, which was established at 29 U. Six controls had positive ELISA with negative Western blots and IIF assays. (B) ELISA results of the data shown in A. The upper limit of normal in the assay is 29 U. Sera confirmed to have anti-hLAMP-2 antibodies by the other two assays are in red, whereas those in which positive ELISA was not confirmed because ELISA and IIF assays were negative are in green. (C) Measurement of antibodies to hLAMP-2 by ELISA and Western blot using hLAMP-2/GST FP and IIF on ldlD/hLAMP-2H cells was compared using a panel of 41 sera selected to cover a range of positive and negative values. The panel consisted of 16 sera from patients with AAV (11 with active disease and 5 in remission), 15 controls with other renal diseases, and 10 healthy controls. The assays were graded positive (red), low positive (Western and IIF), borderline (ELISA) (orange), or negative (green). The figure illustrates the strong concordance among results from the three assays. Sera were considered to have antibodies to LAMP-2 when ≥2 assays were positive. Abbreviations: TX, renal transplant; MGN, membranous nephropathy; IgA GN, IgA nephropathy; MPGN, membranoproliferative GN; TIN, tubulointerstitial nephritis.
Figure 5.
Antibodies to hLAMP-2 in patients with AAV. (A) ELISA results from patients presenting with active piFNGN/AAV from Vienna (_n_=14), Groningen (_n_=44), and Cambridge (_n_=33). The upper limit of normal is 29 and the shaded area between 22 and 29 indicates borderline. Sera confirmed to have anti-hLAMP-2 antibodies by the other two assays are in red, whereas those without anti-hLAMP-2 antibodies are shown in green. Both ELISA and IIF assays were negative in sera in green with positive ELISA. (B) None of the 30 controls from Vienna with various types of renal disease were judged to have anti-hLAMP-2 antibodies, although 4 patients had isolated positive ELISA with negative Western blots and IIF assays. Two of the 21 SLE patients (9.5%) had anti-hLAMP-2 antibodies and an additional 2 patients had positive ELISA not confirmed by the other two assays. (C) Proportion of patients the Vienna, Groningen, and Cambridge cohorts with active piFNGN/AAV with antibodies to LAMP-2. Results are shown separately for untreated patients at presentation, patients presenting after immunosuppressive treatment had been started, and sera taken during relapse. The overall frequency of antibodies to LAMP-2 at presentation was 82% and was higher in untreated patients than those already on treatment. This difference was significant for the whole group (_P_=0.0071, Fisher's exact text) and for the Groningen cohort (_P_=0.0236, Fisher’s exact test).
Figure 6.
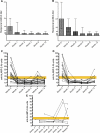
ANCA and anti-hLAMP-2 antibody titers in patients with AAV. (A and B) Sequential ANCA titers expressed as median and interquartile range in the 43 patients in the Groningen cohort followed for 12 months. ANCA titers decreased rapidly after the induction of treatment. The patients are separated into those who had PR3-ANCA (_n_=25; A and C) and MPO-ANCA (_n_=18; B and D). (C and D) Sequential titers of antibodies to hLAMP-2 measured by ELISA in the 43 patients in the Groningen cohort followed for 12 months. The upper limit of normal is 29 U and the yellow bar indicates the borderline positive. The patients are separated into those who had PR3-ANCA (_n_=25) and MPO-ANCA (_n_=18). Anti-hLAMP-2 antibodies became undetectable in <1 month in all but one of the patients. They became undetectable in all 37 positive patients, irrespective of the presence or absence of antibodies to either (C) PR3 or (D) MPO. (E) Sequential titers of antibodies to hLAMP-2 measured by ELISA in six patients from Groningen in the months preceding clinical relapse. The upper limit of normal is 29 U and the yellow bar indicates the borderline positive. Sera from four patients had detectable antibodies to hLAMP-2 before clinical relapse.
Comment in
- Anti-LAMP-2 autoantibodies in ANCA-associated pauci-immune glomerulonephritis.
Flint SM, Savage CO. Flint SM, et al. J Am Soc Nephrol. 2012 Mar;23(3):378-80. doi: 10.1681/ASN.2012010065. Epub 2012 Feb 9. J Am Soc Nephrol. 2012. PMID: 22323640 No abstract available.
Similar articles
- Autoantibodies to hLAMP-2 in ANCA-negative pauci-immune focal necrotizing GN.
Peschel A, Basu N, Benharkou A, Brandes R, Brown M, Rees AJ, Kain R. Peschel A, et al. J Am Soc Nephrol. 2014 Mar;25(3):455-63. doi: 10.1681/ASN.2013030320. Epub 2013 Nov 7. J Am Soc Nephrol. 2014. PMID: 24203998 Free PMC article. - What is the evidence for antibodies to LAMP-2 in the pathogenesis of ANCA associated small vessel vasculitis?
Kain R, Rees AJ. Kain R, et al. Curr Opin Rheumatol. 2013 Jan;25(1):26-34. doi: 10.1097/BOR.0b013e32835b4f8f. Curr Opin Rheumatol. 2013. PMID: 23169102 Review. - Autoantibodies Against Lysosome Associated Membrane Protein-2 (LAMP-2) in Pediatric Chronic Primary Systemic Vasculitis.
Gibson KM, Kain R, Luqmani RA, Ross CJ, Cabral DA, Brown KL. Gibson KM, et al. Front Immunol. 2021 Feb 3;11:624758. doi: 10.3389/fimmu.2020.624758. eCollection 2020. Front Immunol. 2021. PMID: 33613565 Free PMC article. Clinical Trial. - Clinical significance of positive anti-neutrophil cytoplasmic antibodies without evidence of anti-neutrophil cytoplasmic antibodies-associated vasculitis.
Bornstein G, Ben-Zvi I, Furie N, Grossman C. Bornstein G, et al. Int J Rheum Dis. 2019 May;22(5):940-945. doi: 10.1111/1756-185X.13483. Epub 2019 Feb 6. Int J Rheum Dis. 2019. PMID: 30729688 - L29. Relevance of anti-LAMP-2 in vasculitis: why the controversy.
Kain R. Kain R. Presse Med. 2013 Apr;42(4 Pt 2):584-8. doi: 10.1016/j.lpm.2013.01.029. Epub 2013 Feb 28. Presse Med. 2013. PMID: 23453500 Review. No abstract available.
Cited by
- ANCA-Negative Pauci-Immune Glomerulonephritis: A Review.
Juanet C, Hassi I, Koirala A. Juanet C, et al. Glomerular Dis. 2024 Oct 11;4(1):189-199. doi: 10.1159/000541792. eCollection 2024 Jan-Dec. Glomerular Dis. 2024. PMID: 39507613 Free PMC article. Review. - Clinicopathological characteristics and renal outcomes of adult patients with pauci-immune necrotizing glomerulonephritis according to ANCA status.
Elahi T, Ahmed S, Mubarak M, Ahmed E. Elahi T, et al. Clin Rheumatol. 2024 Aug;43(8):2669-2678. doi: 10.1007/s10067-024-07047-7. Epub 2024 Jul 2. Clin Rheumatol. 2024. PMID: 38954279 - New Biomarkers for Systemic Necrotizing Vasculitides.
Shumnalieva R, Ermencheva P, Kotov G, Parvova-Hristova I, Bakopoulou K, Kaouri IE, Mileva N, Velikova T. Shumnalieva R, et al. J Clin Med. 2024 Apr 13;13(8):2264. doi: 10.3390/jcm13082264. J Clin Med. 2024. PMID: 38673537 Free PMC article. Review. - Anti-LAMP-2 Antibody Seropositivity in Children with Primary Systemic Vasculitis Affecting Medium- and Large-Sized Vessels.
Akbaba TH, Toor KK, Mann SK, Gibson KM, Alfaro GA, Balci-Peynircioglu B, Cabral DA, Morishita KA, Brown KL; PedVas Investigator’s Network. Akbaba TH, et al. Int J Mol Sci. 2024 Mar 28;25(7):3771. doi: 10.3390/ijms25073771. Int J Mol Sci. 2024. PMID: 38612581 Free PMC article.
References
- Jennette JC, Falk RJ: Small-vessel vasculitis. N Engl J Med 337: 1512–1523, 1997 - PubMed
- Morgan MD, Harper L, Williams J, Savage C: Anti-neutrophil cytoplasm-associated glomerulonephritis. J Am Soc Nephrol 17: 1224–1234, 2006 - PubMed
- Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, Höglund P, Jayne D, Luqmani R, Mahr A, Mukhtyar C, Pusey C, Rasmussen N, Stegeman C, Walsh M, Westman K; European Vasculitis Study Group: Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 70: 488–494, 2011 - PubMed
- Jennette JC, Xiao H, Falk RJ: Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol 17: 1235–1242, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous