Identification of Akt interaction protein PHF20/TZP that transcriptionally regulates p53 - PubMed (original) (raw)
Identification of Akt interaction protein PHF20/TZP that transcriptionally regulates p53
Sungman Park et al. J Biol Chem. 2012.
Retraction in
- Identification of Akt interaction protein PHF20/TZP that transcriptionally regulates p53.
Park S, Kim D, Dan HC, Chen H, Testa JR, Cheng JQ. Park S, et al. J Biol Chem. 2016 Oct 21;291(43):22852. doi: 10.1074/jbc.A111.333922. J Biol Chem. 2016. PMID: 27825090 Free PMC article. No abstract available.
Abstract
Akt regulates a diverse array of cellular functions, including cell survival, proliferation, differentiation, and metabolism. Although a number of molecules have been identified as upstream regulators and downstream targets of Akt, the mechanisms by which Akt regulates these cellular processes remain elusive. Here, we demonstrate that a novel transcription factor, PHF20/TZP (referring to Tudor and zinc finger domain containing protein), binds to Akt and induces p53 expression at the transcription level. Knockdown of PHF20 significantly reduces p53. PHF20 inhibits cell growth, DNA synthesis, and cell survival. Akt phosphorylates PHF20 at Ser(291) in vitro and in vivo, which results in its translocation from the nucleus to the cytoplasm and attenuation of PHF20 function. These data indicate that PHF20 is a substrate of Akt and plays a role in Akt cell survival/growth signaling.
Figures
FIGURE 1.
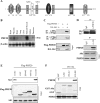
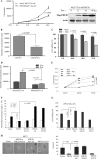
PHF20 interacts with Akt. A, schematic structure of PHF20. PHF20 contains two Tudor, an AT-hook, a C2H2 zinc finger, and a PHD finger domain, three nuclear localization signals (NLS), as well as two putative Akt phosphorylation consensus sites. B, Northern blot analysis shows the expression of PHF20 in multiple human tissues. C, PHF20 binds to Akt. HEK293 cells were co-transfected with FLAG-PHF20 and HA-Akt. After incubation for 48 h, cells were lysed, immunoprecipitated (IP), and immunoblotted (IB) with the indicated antibodies. D, interaction of PHF20 with Akt at endogenous protein levels. Immunoprecipitation and immunoblotting analysis were performed with indicated antibodies in HCT116 cells. E, C-terminal region of PHF20 binds to Akt. MCF7 cells were transfected with the different truncation mutants of FLAG-PHF20, lysed, and immunoprecipitated with FLAG antibody. The immunoprecipitates were immunoblotted with Akt antibody (top). Middle and bottom panels show expression of FLAG-PHF20 and Akt, respectively. F, C-terminal domain of Akt interacts with PHF20. GST pulldown assay was performed by incubation of GST, GST-fused Akt proteins, with in vitro translated PHF20. The pulldown products were immunoblotted with PHF20 antibody (top). The middle panel is Coomassie Blue staining (CBS) and the bottom panel is immunoblotting of the in vitro translated products with PHF20 antibody.
FIGURE 2.
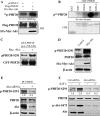
Akt phosphorylates PHF20 in vitro and in vivo. A, PHF20 was phosphorylated by Akt in vivo. In vivo labeling was performed as described under “Experimental Procedures.” Briefly, HEK293 cells were co-transfected with FLAG-PHF20 and constitutively active HA-Myr-Akt or pcDNA3 and labeled with [32P]orthophosphate (0.5 mCi/ml) for 4 h. Cell lysates were immunoprecipitated with FLAG antibody and separated by SDS-PAGE and transferred to membrane. The phosphorylated PHF20 band was examined by autoradiography (top). Middle and bottom panels show the expression of transfected plasmids. B and C, Akt phosphorylates PHF20 in vitro. In vitro kinase assay was performed by incubation of recombinant active Akt with GST-fused PHF20 amino acids 200–280 (Ser265) and 270–330 (Ser291) and their mutant forms. The relative amount of incorporated radioactivity was determined by autoradiography (B) and phospho-PHF20-Ser291 antibody (C). D, Akt phosphorylates PHF20-Ser291 in vivo. HCT116 cells were transfected with constitutively active HA-Myr-Akt or pcDNA3. After incubation of 48 h, cell lysates were immunoblotted with indicated antibodies. E and F, knockdown of Akt reduces phospho-PHF20-Ser291. MDA-MB-468 (E) and MCF7 (F) cells were transfected with Akt siRNA or control siRNA. After 72 h, cell lysates were immunoblotted with indicated antibodies (E). F shows that Akt siRNA or control siRNA-treated MCF7 cells were serum-starved for 12 h, stimulated with IGF1 for 2 h and then immunoblotted with indicated antibodies. WB, Western blot; CBS, Coomassie Blue staining; IP, immunoprecipitation.
FIGURE 3.
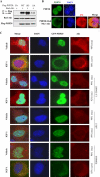
Akt induces PHF20 translocation from the nucleus to the cytoplasm. A, phosphomimic PHF20-S291D but not nonphosphorylatable PHF20-S291A preferentially binds to Akt. MCF7 cells were transfected with indicated plasmids, immunoprecipitated (IP) with FLAG antibody, and detected with Akt antibody (top). Middle and bottom panels show the expression of the transfected plasmids. IB, immunoblot. B, PHF20 localizes in the nucleus, and Akt induces PHF20 nuclear-cytoplasm translocation. MCF7 cells were transfected with FLAG-PHF20 together with/without Red-myr-Akt. After 48 h, cells were stained with FITC-conjugated FLAG antibody and DAPI. C, PI3K or Akt inhibitors but not MEK inhibitor blocked IGF1-induced PHF20 nuclear-cytoplasm translocation. MCF7 cells were transfected with GFP-PHF20. After 36 h, cells were deprived from serum for 12 h and treated indicated drugs for 30 min prior to IGF1 stimulation for 1 h. Cells were stained with Akt antibody and Texas Red-conjugated secondary antibody.
FIGURE 4.
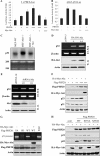
PHF20 binds to DNA and transcriptionally up-regulates p53. A, association of PHF20 with chromatin. Cells were resuspended in buffer A with Nonidet P-40 (0.1%). Nuclei were collected by low speed centrifugation. Insoluble chromatin pellet (P3) was collected by centrifugation and resuspended in SDS-loading buffer. Release of chromatin-bound PHF20 proteins was detected following treatment of the cell nuclei with micrococcal nuclease (MNase). B, electrophoretic mobility shift assay was performed as described under “Experimental Procedures.” C, PHF20 possesses transcription factor activity. Three repeats of PHF20-binding consensus binding sequence were cloned to pGL3-basic vector (3 × PBCS-Luc), which was co-transfected into MCF7 cells with indicated plasmids. Following incubation for 36 h, luciferase activity was measured and normalized to β-galactosidase. Results are the mean ± S.E. of three independent experiments performed in triplicate. D and E, PHF20 induces p53. MCF7/Tet-Off inducible PHF20 cells were cultured in the media without doxycycline (Dox) for the indicated time and then were immunoblotted with indicated antibodies (D) and were subjected to Northern blot analysis probed with p53 and β-actin (E). F, knockdown of PHF20 reduces p53 expression. MCF7 and HCT116 were transfected with PHF20 siRNA or control siRNA and subjected to immunoblotting (left) and Northern blot (right) analysis. G, PHF20 induces p53 promoter activity. Luciferase report assay was performed as described in C in MCF7 cells that were transfected with indicated plasmids. H, ChIP assay shows that PHF20 directly binds to p53 promoter in vivo. ChIP assay was performed with PHF20 antibody in HCT116 cells, and PCR was carried out using the primers spanning two PHF20-binding sites in the p53 promoter. IP, immunoprecipitation.
FIGURE 5.
Akt inhibits PHF20-induced p53. A and B, constitutively active Akt inhibits PHF20 transcriptional activity. Luciferase reporter assay was performed in MCF7 cells that were transfected with 3×PBCS-Luc (A) or pLG3-p53-Luc (B) together with other indicated plasmids. C, expression of Akt repressed p53 mRNA level. Akt-null MEFs were transfected with/without Akt and subjected to Northern blot analysis (top). Bottom panel is loading control. D and E, inhibition of Akt increases p53 expression. MCF7 (D) and HCT116 (E) cells were transfected with dominant-negative Akt or Akt shRNA, respectively, and then subjected to semi-quantitative RT-PCR and Western blot (W.B.) analysis. F, Akt represses PHF20-induced p53 expression. HCT116 cells were transfected with indicated plasmids and then immunoblotted with indicated antibodies. G and H, inhibition of PHF20-induced p53 by Akt depends on phosphorylation of PHF20-Ser291. MCF7 cells were transfected with indicated plasmids and then subjected to ChIP (top panel of G) and immunoblotting (panels 2–4 of G and H) analyses. Cont, control.
FIGURE 6.
PHF20 exhibited cell growth inhibitory function that was overridden by Akt. A–C, PHF20 inhibited cell proliferation, DNA synthesis, and cell survival. MCF7/Tet-Off PHF20 and MCF7/Tet-Off control cells were seeded in 24-well plate. After withdrawal of doxycycline (Dox) for indicated times, cell growth (A), DNA synthesis (B), and cell survival (C) were assayed with accounting cell number, thymidine incorporation, and MTS as described under “Experimental Procedures.” Right panel of A shows expression of PHF20. D, PHF20 reduced DNA synthesis in HCT116 cells. E–G, Akt inhibits PHF20 cellular function. MCF7/Tet-Off PHF20 and MCF7/Tet-Off control cells were transfected with myr-Akt and cultured in the absence and presence of doxycycline. Cell number was accounted at indicated times (E). F and G, MCF7 cells were transfected with indicated plasmids and then assayed for DNA synthesis (F) and cell viability (G). H, effects of PHF20 and Akt phosphorylation of PHF20 on cell anchorage-independent growth. MCF7 cells were transfected with indicated plasmids and then grown in soft agar for 3 weeks (left panel). The colony was accounted and quantified in three plates/transfectant (right panel).
FIGURE 7.
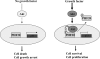
Diagram depicting the regulation of PHF20 by Akt. In the absence of cell growth and survival signals, PHF20 locates in the nucleus where it binds to DNA and regulates p53 and other gene expression leading to cell growth arrest and cell death (left panel). Once Akt is activated, Akt phosphorylates PHF20Ser291 in the nucleus, which results in PHF20 nuclear-cytoplasmic translocation (right panel), which results in cell survival and proliferation.
Similar articles
- PHF20 is an effector protein of p53 double lysine methylation that stabilizes and activates p53.
Cui G, Park S, Badeaux AI, Kim D, Lee J, Thompson JR, Yan F, Kaneko S, Yuan Z, Botuyan MV, Bedford MT, Cheng JQ, Mer G. Cui G, et al. Nat Struct Mol Biol. 2012 Sep;19(9):916-24. doi: 10.1038/nsmb.2353. Epub 2012 Aug 5. Nat Struct Mol Biol. 2012. PMID: 22864287 Free PMC article. - PHF20 Readers Link Methylation of Histone H3K4 and p53 with H4K16 Acetylation.
Klein BJ, Wang X, Cui G, Yuan C, Botuyan MV, Lin K, Lu Y, Wang X, Zhao Y, Bruns CJ, Mer G, Shi X, Kutateladze TG. Klein BJ, et al. Cell Rep. 2016 Oct 18;17(4):1158-1170. doi: 10.1016/j.celrep.2016.09.056. Cell Rep. 2016. PMID: 27760318 Free PMC article. - PKB-mediated PHF20 phosphorylation on Ser291 is required for p53 function in DNA damage.
Li Y, Park J, Piao L, Kong G, Kim Y, Park KA, Zhang T, Hong J, Hur GM, Seok JH, Choi SW, Yoo BC, Hemmings BA, Brazil DP, Kim SH, Park J. Li Y, et al. Cell Signal. 2013 Jan;25(1):74-84. doi: 10.1016/j.cellsig.2012.09.009. Epub 2012 Sep 11. Cell Signal. 2013. PMID: 22975685 - Crystal structures of the Tudor domains of human PHF20 reveal novel structural variations on the Royal Family of proteins.
Adams-Cioaba MA, Li Z, Tempel W, Guo Y, Bian C, Li Y, Lam R, Min J. Adams-Cioaba MA, et al. FEBS Lett. 2012 Mar 23;586(6):859-65. doi: 10.1016/j.febslet.2012.02.012. Epub 2012 Feb 24. FEBS Lett. 2012. PMID: 22449972 - p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment.
Feng Z. Feng Z. Cold Spring Harb Perspect Biol. 2010 Feb;2(2):a001057. doi: 10.1101/cshperspect.a001057. Cold Spring Harb Perspect Biol. 2010. PMID: 20182617 Free PMC article. Review.
Cited by
- Metformin synergistically increases the anticancer effects of lapatinib through induction of apoptosis and modulation of Akt/AMPK pathway in SK-BR3 breast cancer cell line.
Neamati D, Khedri A, Aberomand M, Hemmati AA, Mohammadzadeh M, Akbari Baghbani K, Mohammadzadeh G. Neamati D, et al. Iran J Basic Med Sci. 2021 Nov;24(11):1529-1537. doi: 10.22038/IJBMS.2021.58825.13069. Iran J Basic Med Sci. 2021. PMID: 35317106 Free PMC article. - Clinical Development of AKT Inhibitors and Associated Predictive Biomarkers to Guide Patient Treatment in Cancer Medicine.
Coleman N, Moyers JT, Harbery A, Vivanco I, Yap TA. Coleman N, et al. Pharmgenomics Pers Med. 2021 Nov 25;14:1517-1535. doi: 10.2147/PGPM.S305068. eCollection 2021. Pharmgenomics Pers Med. 2021. PMID: 34858045 Free PMC article. Review. - Targeting AKT for cancer therapy.
Shariati M, Meric-Bernstam F. Shariati M, et al. Expert Opin Investig Drugs. 2019 Nov;28(11):977-988. doi: 10.1080/13543784.2019.1676726. Epub 2019 Oct 12. Expert Opin Investig Drugs. 2019. PMID: 31594388 Free PMC article. Review. - Chaperone-E3 Ligase Complex HSP70-CHIP Mediates Ubiquitination of Ribosomal Protein S3.
Hwang I, Cho SW, Ahn JY. Hwang I, et al. Int J Mol Sci. 2018 Sep 12;19(9):2723. doi: 10.3390/ijms19092723. Int J Mol Sci. 2018. PMID: 30213050 Free PMC article. - Adaptations to Endurance and Strength Training.
Hughes DC, Ellefsen S, Baar K. Hughes DC, et al. Cold Spring Harb Perspect Med. 2018 Jun 1;8(6):a029769. doi: 10.1101/cshperspect.a029769. Cold Spring Harb Perspect Med. 2018. PMID: 28490537 Free PMC article. Review.
References
- Coffer P. J., Woodgett J. R. (1991) Molecular cloning and characterization of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur. J. Biochem. 201, 475–481 - PubMed
- Bellacosa A., Testa J. R., Staal S. P., Tsichlis P. N. (1991) A retroviral oncogene, akt, encoding a serine threonine kinase containing an SH2-like region. Science 254, 274–277 - PubMed
- Cheng J. Q., Godwin A. K., Bellacosa A., Taguchi T., Franke T. F., Hamilton T. C., Tsichlis P. N., Testa J. R. (1992) AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl. Acad. Sci. U.S.A. 89, 9267–9271 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA137041/CA/NCI NIH HHS/United States
- R01 CA077429/CA/NCI NIH HHS/United States
- P30 CA076292/CA/NCI NIH HHS/United States
- CA77429/CA/NCI NIH HHS/United States
- CA137041/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous