An animal model of MYC-driven medulloblastoma - PubMed (original) (raw)
. 2012 Feb 14;21(2):155-67.
doi: 10.1016/j.ccr.2011.12.021.
Colin E Moore, Jun Wang, Alok K Tewari, Alexey Eroshkin, Yoon-Jae Cho, Hendrik Witt, Andrey Korshunov, Tracy-Ann Read, Julia L Sun, Earlene M Schmitt, C Ryan Miller, Anne F Buckley, Roger E McLendon, Thomas F Westbrook, Paul A Northcott, Michael D Taylor, Stefan M Pfister, Phillip G Febbo, Robert J Wechsler-Reya
Affiliations
- PMID: 22340590
- PMCID: PMC3285431
- DOI: 10.1016/j.ccr.2011.12.021
An animal model of MYC-driven medulloblastoma
Yanxin Pei et al. Cancer Cell. 2012.
Abstract
Medulloblastoma (MB) is the most common malignant brain tumor in children. Patients whose tumors exhibit overexpression or amplification of the MYC oncogene (c-MYC) usually have an extremely poor prognosis, but there are no animal models of this subtype of the disease. Here, we show that cerebellar stem cells expressing Myc and mutant Trp53 (p53) generate aggressive tumors following orthotopic transplantation. These tumors consist of large, pleiomorphic cells and resemble human MYC-driven MB at a molecular level. Notably, antagonists of PI3K/mTOR signaling, but not Hedgehog signaling, inhibit growth of tumor cells. These findings suggest that cerebellar stem cells can give rise to MYC-driven MB and identify a novel model that can be used to test therapies for this devastating disease.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
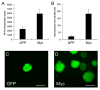
Figure 1. Myc promotes proliferation of cerebellar stem cells in vitro
(A), Prom1+Lin− cells sorted from cerebella of 5–7 day-old mice were infected with _Myc_-IRES-GFP or control (GFP only) viruses for 48h, pulsed with tritiated thymidine (3H-Td) and cultured overnight before being assayed for 3H-Td incorporation. Data represent means of triplicate samples ± SEM. (B–D) Prom1+Lin− cells infected with _Myc_-IRES-GFP or control viruses were cultured at low density in the presence of EGF and bFGF for 7 days. Representative fields are shown in panels C and D (scale bars = 100 μm). The number of GFP+ neurospheres is quantified in B; data represent means of triplicate samples ± SEM. The infection efficiency was 80% with _Myc_-IRES-GFP and 90% with control retrovirus. See also Figure S1.
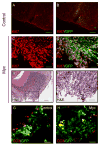
Figure 2. _Myc_-infected stem cells give rise to transient hyperplastic lesions following transplantation
Prom1+Lin− cells were infected with _Myc_-IRES-GFP or control retroviruss for 20h and then transplanted into the cerebellum of NSG mice. Hosts were sacrificed after 2.5 weeks. Frozen sections from mice that received GFP-infected (A–B) or Myc infected cells (C–F) were stained with anti-Ki67 antibodies (A–D) or H&E (E, F). Note the large mass of proliferating (Ki67+) cells seen in animals that received _Myc_-infected cells (C, D). Box in E corresponds to high-power field shown in F. Panels A–D and F, scale bars = 50 μm; panel E, scale bar = 100 μm. (G–H) Prom1+ cells were infected with _Myc_-ires-GFP or control-GFP viruses for 20h and then transplanted into the cerebellum of NSG hosts. Mice were sacrificed after 2 weeks. Frozen sections from mice that received control (G) or Myc infected cells (H) were stained with antibodies specific for cleaved caspase-3 (CC3) to detect apoptotic cells. Scale bars = 50 μm. See also Figure S2.
Figure 3. Overexpression of Myc and inactivation of p53 transforms cerebellar stem cells into tumors
Prom1+Lin− cells were infected with Myc + DNp53 retroviruses, Myc alone or DNp53 alone for 20h, and transplanted into cerebella of NSG mice. Animals were sacrificed when they developed symptoms. (A), Whole mount image of tumor, with GFP expression originating from DNp53 retrovirus. (B) Survival curve of animals receiving 5 x104 cells infected with Myc viruses (blue line), DNp53 viruses (green line) or Myc + DNp53 viruses (red line) (median survival 48 days). (C–H) Sections of tumor tissue from animals transplanted with cells expressing Myc and DNp53 (C–E) or from Ptch1 mutant mice (F–H) were stained with hematoxylin and eosin. For C and F scale bars = 100 μm; for D, E, G and H, scale bars = 50 μm. Boxes in panels C and F refer to panels D and G, respectively. Asterisk in C shows an area of necrosis, and asterisk in D shows a large tumor cell with marked nuclear atypia (anaplasia). Horizontal arrows in D and E show prominent nuclear molding. Arrowheads in D, G and H show mitotic figures. Vertical arrows in E and H show normal granule neurons in the internal granule layer (igl); the majority of tumor cells in E are much larger than these cells, whereas those in H are approximately the same size. See also Figure S3.
Figure 4. MP tumors exhibit characteristics of human MB
Cryosections from MP tumors were stained with H&E (A) or with antibodies specific for Ki67 (B), Nestin (C), Tuj1 (D), GFAP (E) or BAF47/Ini1 (F). Panels A–E represent adjacent sections. Scale bars = 50 μm. See also Figure S4.
Figure 5. Myc is required for continued growth of MP tumors
(A) Strategy for generating Tet-regulatable MP tumors. (B–F) Bioluminescent imaging of animals at 1, 2 and 3 weeks after tumor cells transplantation. Panel B shows representative images of 4 animals from each group at each time point (X’s denote animals that died before they could be imaged). Graphs on right show mean percent increase in bioluminescence for all animals in the group (with the 1-week signal for each animal set at 100%). In the top graph, the 1- and 2-week time points represent the average signal intensity for all 14 animals; the 3-week time point (marked by asterisk) represents the average for the 3 animals that remained alive at the time of imaging. (C–E) H&E-stained cerebellar sections from representative animals in Groups 1 (C), 2 (D) and 3 (E) three weeks after transplantation. Arrows in D and E point to injection site. Scale bars = 250 μm. (F) Survival curve (Groups 1 and 2, n=14; Group 3, n=12).
Figure 6. MP tumors resemble human MYC-driven MB
A, Gene expression profiles of Myc/DNp53 tumors from Prom1+/Lin− cells (MP-pl2-6) or from Prom1+ cells (MP-P3-7) and Ptch1 mutant (Ptc1-4) tumors were compared to signatures generated from human MB subtypes: WNT (blue), SHH (red), and Group C/D (green). Each murine tumor was assigned a score denoting its similarity to each subtype of human tumor (for details see Human Tumor Analysis Supplement and Table S1). (B–G) Ptch1 and MP tumors were stained with antibodies specific for secreted frizzled-related protein 1 (SFRP1, a marker for SHH tumors), Natriuretic Peptide Receptor C (NPR3, a marker for Group C tumors), or Potassium voltage-gated channel, shaker-related subfamily, member 1 (KCNA1, a marker for Group D tumors). Scale bars = 100 μm. See also Figure S5 and Table S1.
Figure 7. MP tumors are molecularly distinct from stem cells and from Ptch1 tumors
(A) Principle components analysis (PCA). Three PCA coordinates describe 55.2% of total data variation (PC1 – 27.2%, PC2 – 19.8% and PC3 – 8.23%). Green, MP tumors derived from Prom1+Lin− cells; purple, MP tumors derived from Prom1+ cells; blue, Ptch1 tumors; red, normal stem cells (NSCs). (B) Unsupervised hierarchical clustering analysis. Each column represents a distinct sample and each row represents an individual gene. The normalized (log2) and standardized (each sample to mean signal = 0 and standard deviation = 1) level of gene expression is denoted by color (green = low, dark = intermediate, red = high) as indicated in the gradient at the bottom. (C–F) Genes differentially expressed between MP tumors and Ptch1 tumors were subjected to NextBio analysis, to identify Biogroups and Studies that contain similar genes. Representative Biogroups (C–D, E) and Studies (F) are shown. Venn diagrams show the number of common and unique genes in both sets. Bars on the right show the significance of overlap between gene subsets (the scale of the bar is measured in −log(p-value), so taller the bar, the higher the significance of the gene overlap). Whereas each Biogroup is represented by a single list of genes, signature genes from Studies have two lists, one for up-regulated and one for down-regulated genes. Thus, Biogroup comparisons consist of just two graphs, whereas comparisons to Studies consist of four graphs. See also Tables S2–6.
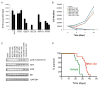
Figure 8. Growth of MP tumor cells is inhibited by antagonists of PI3K/mTOR signaling
(A) Effects of inhibitors on short-term proliferation. MP tumor cells were cultured in serum-free media containing no additive (ø), vehicle (DMSO), cyclopamine (0.1, 1, 2.5 μM), 10058-F4 (10, 25, 100 μM), BEZ-235 (0.2, 1, 5 μM), BKM-120 (0.2, 1, 5μM), or RAD-001 (0.2, 1, 5μM). For each inhibitor, columns are ordered from lowest to highest concentration. After 48h, cells were pulsed with 3H-Td and cultured overnight before being assayed for 3H-Td incorporation. Data represent means of triplicate samples ± SEM. (B) Effects on long-term growth. Tumor cells were cultured for 3, 7 or 14 days in the presence of different doses of inhibitors and cell number was counted at the indicated time points. (C) Effects on PI3K signaling. Tumor cells were treated with DMSO, BEZ-235 (5uM, 1uM), BKM-120 (5uM, 1uM), RAD-001(5uM, 1uM) or cyclopamine (2.5uM, 1uM) for 3 hr. Cells were lysed and protein was analyzed for phosphorylation of AKT and S6 (pAKT and pS6) or for GAPDH by Western blotting. (D) Effects on tumor growth in vivo. 500 MP tumor cells were re-transplanted into naïve NSG mice. After 7 days, mice were imaged for luciferase activity and separated into two groups randomly. Mice in Group 1 were treated with vehicle (0.5% methyl-cellulose) and those in Group 2 were treated with BKM-120 (30mg/kg per day) by oral gavage until they developed symptoms. BMK-120 treatment significantly prolonged survival compared to vehicle (p=0.001). See also Figure S6.
Comment in
- Three down and one to go: modeling medulloblastoma subgroups.
Eberhart CG. Eberhart CG. Cancer Cell. 2012 Feb 14;21(2):137-8. doi: 10.1016/j.ccr.2012.01.013. Cancer Cell. 2012. PMID: 22340583 Free PMC article.
Similar articles
- A Novel Combination Approach Targeting an Enhanced Protein Synthesis Pathway in MYC-driven (Group 3) Medulloblastoma.
Chaturvedi NK, Kling MJ, Griggs CN, Kesherwani V, Shukla M, McIntyre EM, Ray S, Liu Y, McGuire TR, Sharp JG, Band H, Joshi SS, Coulter DW. Chaturvedi NK, et al. Mol Cancer Ther. 2020 Jun;19(6):1351-1362. doi: 10.1158/1535-7163.MCT-19-0996. Epub 2020 May 5. Mol Cancer Ther. 2020. PMID: 32371591 - MYC Drives Group 3 Medulloblastoma through Transformation of Sox2+ Astrocyte Progenitor Cells.
Tao R, Murad N, Xu Z, Zhang P, Okonechnikov K, Kool M, Rivero-Hinojosa S, Lazarski C, Zheng P, Liu Y, Eberhart CG, Rood BR, Packer R, Pei Y. Tao R, et al. Cancer Res. 2019 Apr 15;79(8):1967-1980. doi: 10.1158/0008-5472.CAN-18-1787. Epub 2019 Mar 12. Cancer Res. 2019. PMID: 30862721 Free PMC article. - TORC1/2 kinase inhibition depletes glutathione and synergizes with carboplatin to suppress the growth of MYC-driven medulloblastoma.
Maynard RE, Poore B, Hanaford AR, Pham K, James M, Alt J, Park Y, Slusher BS, Tamayo P, Mesirov J, Archer TC, Pomeroy SL, Eberhart CG, Raabe EH. Maynard RE, et al. Cancer Lett. 2021 Apr 28;504:137-145. doi: 10.1016/j.canlet.2021.02.001. Epub 2021 Feb 8. Cancer Lett. 2021. PMID: 33571541 Free PMC article. - Targeting mTOR as a Therapeutic Approach in Medulloblastoma.
Aldaregia J, Odriozola A, Matheu A, Garcia I. Aldaregia J, et al. Int J Mol Sci. 2018 Jun 22;19(7):1838. doi: 10.3390/ijms19071838. Int J Mol Sci. 2018. PMID: 29932116 Free PMC article. Review. - A Proteogenomic Approach to Understanding MYC Function in Metastatic Medulloblastoma Tumors.
Staal JA, Pei Y, Rood BR. Staal JA, et al. Int J Mol Sci. 2016 Oct 19;17(10):1744. doi: 10.3390/ijms17101744. Int J Mol Sci. 2016. PMID: 27775567 Free PMC article. Review.
Cited by
- Molecular Classification of Medulloblastoma.
Kijima N, Kanemura Y. Kijima N, et al. Neurol Med Chir (Tokyo). 2016 Nov 15;56(11):687-697. doi: 10.2176/nmc.ra.2016-0016. Epub 2016 May 26. Neurol Med Chir (Tokyo). 2016. PMID: 27238212 Free PMC article. Review. - Children's Oncology Group's 2013 blueprint for research: central nervous system tumors.
Gajjar A, Packer RJ, Foreman NK, Cohen K, Haas-Kogan D, Merchant TE; COG Brain Tumor Committee. Gajjar A, et al. Pediatr Blood Cancer. 2013 Jun;60(6):1022-6. doi: 10.1002/pbc.24427. Epub 2012 Dec 19. Pediatr Blood Cancer. 2013. PMID: 23255213 Free PMC article. Review. - Orally bioavailable glutamine antagonist prodrug JHU-083 penetrates mouse brain and suppresses the growth of MYC-driven medulloblastoma.
Hanaford AR, Alt J, Rais R, Wang SZ, Kaur H, Thorek DLJ, Eberhart CG, Slusher BS, Martin AM, Raabe EH. Hanaford AR, et al. Transl Oncol. 2019 Oct;12(10):1314-1322. doi: 10.1016/j.tranon.2019.05.013. Epub 2019 Jul 21. Transl Oncol. 2019. PMID: 31340195 Free PMC article. - Loss of phosphatase CTDNEP1 potentiates aggressive medulloblastoma by triggering MYC amplification and genomic instability.
Luo Z, Xin D, Liao Y, Berry K, Ogurek S, Zhang F, Zhang L, Zhao C, Rao R, Dong X, Li H, Yu J, Lin Y, Huang G, Xu L, Xin M, Nishinakamura R, Yu J, Kool M, Pfister SM, Roussel MF, Zhou W, Weiss WA, Andreassen P, Lu QR. Luo Z, et al. Nat Commun. 2023 Feb 10;14(1):762. doi: 10.1038/s41467-023-36400-8. Nat Commun. 2023. PMID: 36765089 Free PMC article. - Charting oncogenicity of genes and variants across lineages via multiplexed screens in teratomas.
Parekh U, McDonald D, Dailamy A, Wu Y, Cordes T, Zhang K, Tipps A, Metallo C, Mali P. Parekh U, et al. iScience. 2021 Sep 20;24(10):103149. doi: 10.1016/j.isci.2021.103149. eCollection 2021 Oct 22. iScience. 2021. PMID: 34646987 Free PMC article.
References
- Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006 - PubMed
- Bowman T, Symonds H, Gu L, Yin C, Oren M, Van Dyke T. Tissue-specific inactivation of p53 tumor suppression in the mouse. Genes Dev. 1996;10:826–835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA159859/CA/NCI NIH HHS/United States
- R01 CA159859/CA/NCI NIH HHS/United States
- R01 CA159859-01/CA/NCI NIH HHS/United States
- R01 CA122759-04/CA/NCI NIH HHS/United States
- R01 CA122759/CA/NCI NIH HHS/United States
- CA122759/CA/NCI NIH HHS/United States
- R01 CA122759-03/CA/NCI NIH HHS/United States
- R01 CA159859-02/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous