The sarcomeric protein nebulin: another multifunctional giant in charge of muscle strength optimization - PubMed (original) (raw)
The sarcomeric protein nebulin: another multifunctional giant in charge of muscle strength optimization
Coen A C Ottenheijm et al. Front Physiol. 2012.
Abstract
The sliding filament model of the sarcomere was developed more than half a century ago. This model, consisting only of thin and thick filaments, has been successful in explaining many, but not all, features of skeletal muscle. Work during the 1980s revealed the existence of two additional filaments: the giant filamentous proteins titin and nebulin. Whereas the role of titin rapidly progressed, nebulin's role in muscle structure and function remained long nebulous. An important feature of muscle structure and function that has remained relatively obscure concerns the mechanisms that are involved in regulating thin filament length. Filament length is an important aspect of muscle function as force production is proportional to the amount of overlap between thick and thin filaments. Recent advances, due in part to the generation of nebulin KO models, reveal that nebulin plays an important role in the regulation of thin filament length, most likely by stabilizing F-actin assemblies. Another structural feature of skeletal muscle that has been incompletely understood concerns the mechanisms involved in maintaining Z-disk structure and the regular lateral alignment of adjacent sarcomeres during contraction. Recent studies indicate that nebulin is part of a protein complex that mechanically links adjacent myofibrils. In addition to these structural roles in support of myofibrillar force generation, nebulin has been also shown to regulate directly muscle contraction at the level of individual crossbridges: cycling kinetics and the calcium sensitivity of force producing crossbridges is enhanced in the presence of nebulin. Thus, these recent data all point to nebulin being important for muscle force optimization. Consequently, muscle weakness as the lead symptom develops in the case of patients with nemaline myopathy that have mutations in the nebulin gene. Here, we discuss these important novel insights into the role of nebulin in skeletal muscle function.
Keywords: nebulin; skeletal muscle; thin filament.
Figures
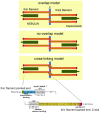
Figure 1
Top: Three schematics of the skeletal muscle sarcomere depicting the three proposed models of nebulin in the Z-disk: the overlap model in which nebulin penetrates the Z-disk creating a ~75 nm wide nebulin overlap zone in the center of the Z-disk; the no-overlap model in which nebulin penetrates the Z-disk by only about 25 nm, and nebulin filaments from neighboring sarcomeres do not overlap; the cross-linking model in which nebulin fully penetrates the Z-disk and cross-links thin filaments from adjacent sarcomeres. Also note that a single nebulin molecule spans most of the length of the thin filament, but that according to recent studies the thin filament pointed-end is nebulin-free. The length of the nebulin-free thin filament extension is variable (as indicated in the drawing by the dotted nebulin filament) and depends on species and muscle type. Bottom: Schematic of the human nebulin sequence. Nebulin has a highly modular structure, with in the central region (M9–M162) seven modular repeats arranged into 22 super-repeats.
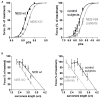
Figure 2
(A) Force–Ca2+ characteristics of murine skinned nebulin-deficient muscle fibers from m. tibialis cranialis (NEB-KO, left panel), and of skinned muscle fibers from m. quadriceps of patients with nebulin-based nemaline myopathy (NEB-NM, right panel). Note that the force generated in response to incubation with incremental increase of [Ca2+] is significantly decreased in both NEB-KO and NEB-NM tissue, resulting in a rightward shift of the force–Ca2+ relationship (Figures adapted from Chandra et al., ; Ottenheijm et al., 2010). (B) Left: the force-sarcomere length relation of murine wt (NEB-wt) fibers has a characteristic force plateau followed by a descending limb. The force-sarcomere length relation of NEB-KO fibers is shifted leftward compared to control fibers, and the force plateau is absent. Right: the force-sarcomere length relation of muscle fibers from NM-NEB patients is shifted to the left and is very similar to that found in NEB-KO fibers (Figures adapted from Ottenheijm et al., 2008).
Similar articles
- New insights into the structural roles of nebulin in skeletal muscle.
Ottenheijm CA, Granzier H. Ottenheijm CA, et al. J Biomed Biotechnol. 2010;2010:968139. doi: 10.1155/2010/968139. Epub 2010 Jun 1. J Biomed Biotechnol. 2010. PMID: 20589077 Free PMC article. Review. - Deleting exon 55 from the nebulin gene induces severe muscle weakness in a mouse model for nemaline myopathy.
Ottenheijm CA, Buck D, de Winter JM, Ferrara C, Piroddi N, Tesi C, Jasper JR, Malik FI, Meng H, Stienen GJ, Beggs AH, Labeit S, Poggesi C, Lawlor MW, Granzier H. Ottenheijm CA, et al. Brain. 2013 Jun;136(Pt 6):1718-31. doi: 10.1093/brain/awt113. Epub 2013 May 28. Brain. 2013. PMID: 23715096 Free PMC article. - Expressing a Z-disk nebulin fragment in nebulin-deficient mouse muscle: effects on muscle structure and function.
Li F, Kolb J, Crudele J, Tonino P, Hourani Z, Smith JE 3rd, Chamberlain JS, Granzier H. Li F, et al. Skelet Muscle. 2020 Jan 28;10(1):2. doi: 10.1186/s13395-019-0219-9. Skelet Muscle. 2020. PMID: 31992366 Free PMC article. - Reduced myofibrillar connectivity and increased Z-disk width in nebulin-deficient skeletal muscle.
Tonino P, Pappas CT, Hudson BD, Labeit S, Gregorio CC, Granzier H. Tonino P, et al. J Cell Sci. 2010 Feb 1;123(Pt 3):384-91. doi: 10.1242/jcs.042234. Epub 2010 Jan 5. J Cell Sci. 2010. PMID: 20053633 Free PMC article. - Nebulin: big protein with big responsibilities.
Yuen M, Ottenheijm CAC. Yuen M, et al. J Muscle Res Cell Motil. 2020 Mar;41(1):103-124. doi: 10.1007/s10974-019-09565-3. Epub 2020 Jan 25. J Muscle Res Cell Motil. 2020. PMID: 31982973 Free PMC article. Review.
Cited by
- Proteomic changes of the bovine blood plasma in response to heat stress in a tropically adapted cattle breed.
Reolon HG, Abduch NG, de Freitas AC, Silva RMO, Fragomeni BO, Lourenco D, Baldi F, de Paz CCP, Stafuzza NB. Reolon HG, et al. Front Genet. 2024 Aug 1;15:1392670. doi: 10.3389/fgene.2024.1392670. eCollection 2024. Front Genet. 2024. PMID: 39149588 Free PMC article. - Characterization of NEB pathogenic variants in patients reveals novel nemaline myopathy disease mechanisms and omecamtiv mecarbil force effects.
Karimi E, Gohlke J, van der Borgh M, Lindqvist J, Hourani Z, Kolb J, Cossette S, Lawlor MW, Ottenheijm C, Granzier H. Karimi E, et al. Acta Neuropathol. 2024 Apr 18;147(1):72. doi: 10.1007/s00401-024-02726-w. Acta Neuropathol. 2024. PMID: 38634969 Free PMC article. - Role of Actin-Binding Proteins in Skeletal Myogenesis.
Nguyen MT, Dash R, Jeong K, Lee W. Nguyen MT, et al. Cells. 2023 Oct 25;12(21):2523. doi: 10.3390/cells12212523. Cells. 2023. PMID: 37947600 Free PMC article. Review. - Nucleus Mechanosensing in Cardiomyocytes.
Coscarella IL, Landim-Vieira M, Rastegarpouyani H, Chase PB, Irianto J, Pinto JR. Coscarella IL, et al. Int J Mol Sci. 2023 Aug 28;24(17):13341. doi: 10.3390/ijms241713341. Int J Mol Sci. 2023. PMID: 37686151 Free PMC article. Review. - The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles.
Szikora S, Görög P, Mihály J. Szikora S, et al. Int J Mol Sci. 2022 May 10;23(10):5306. doi: 10.3390/ijms23105306. Int J Mol Sci. 2022. PMID: 35628117 Free PMC article. Review.
References
- Agrawal P. B., Greenleaf R. S., Tomczak K. K., Lehtokari V. L., Wallgren-Pettersson C., Wallefeld W., Laing N. G., Darras B. T., Maciver S. K., Dormitzer P. R., Beggs A. H. (2007). Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am. J. Hum. Genet. 80, 162–16710.1086/510402 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials