Sex differences in brain proteomes of neuron-specific STAT3-null mice after cerebral ischemia/reperfusion - PubMed (original) (raw)
. 2012 May;121(4):680-92.
doi: 10.1111/j.1471-4159.2012.07721.x. Epub 2012 Mar 28.
Gabriella Casalena, Jia Jia, Rukhsana Sultana, Eugenio Barone, Jian Cai, William M Pierce, Chiara Cini, Cesare Mancuso, Marzia Perluigi, Catherine M Davis, Nabil J Alkayed, D Allan Butterfield
Affiliations
- PMID: 22394374
- PMCID: PMC3325362
- DOI: 10.1111/j.1471-4159.2012.07721.x
Sex differences in brain proteomes of neuron-specific STAT3-null mice after cerebral ischemia/reperfusion
Fabio Di Domenico et al. J Neurochem. 2012 May.
Erratum in
- J Neurochem. 2012 Sep;122(5):1093. Butterfield, Allan D [corrected to Butterfield, D Allan]
Abstract
Signal transduction and activator of transcription-3 (STAT3) plays an important role in neuronal survival, regeneration and repair after brain injury. We previously demonstrated that STAT3 is activated in brain after cerebral ischemia specifically in neurons. The effect was sex-specific and modulated by sex steroids, with higher activation in females than males. In the current study, we used a proteomics approach to identify downstream proteins affected by ischemia in male and female wild-type (WT) and neuron-specific STAT3 knockout (KO) mice. We established four comparison groups based on the transgenic condition and the hemisphere analyzed, respectively. Moreover, the sexual variable was taken into account and male and female animals were analyzed independently. Results support a role for STAT3 in metabolic, synaptic, structural and transcriptional responses to cerebral ischemia, indeed the adaptive response to ischemia/reperfusion injury is delayed in neuronal-specific STAT3 KO mice. The differences observed between males and females emphasize the importance of sex-specific neuronal survival and repair mechanisms, especially those involving antioxidant and energy-related activities, often caused by sex hormones.
© 2012 The Authors. Journal of Neurochemistry © 2012 International Society for Neurochemistry.
Figures
Figure 1
Representative 2D gel of protein identified, by mass spectrometry, with altered levels in WT (left) and STAT3 KO (right) female (top) and male (bottom) ipsilateral vs. contralateral proteomics comparison. O = altered proteins identified in WT animals; △ = altered proteins identified in neuronal-specific STAT3 KO animals;
Figure 2
Representative 2D gel of protein identified, by mass spectrometry, with altered levels in contralateral (left) and ipsilateral (right) female (top) and male (bottom) WT vs. neuronal-specific STAT3 KO proteomics comparison. O = altered proteins identified in contralateral hemisphere; △ = altered proteins identified in ipsilateral hemisphere;
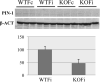
Figure 3
Western blot image and relative graph bar of Pin-1 and β actin protein levels in WT and neuronal-specific STAT3 KO, ispsilateral and contralateral female samples; comparison between WT female ispilateral and neuronal-specific STAT3-KO female ipsilateral samples.
Figure 4
Interactome maps showing direct and indirect relationship between the proteins identified in this study with altered expression (yellow) in presence or absence of STAT3 in neuronal tissue; LEFT: direct and indirect interaction of all the proteins between them, with and trough STAT3, the green lines represent the direct interaction of identified protein and STAT3, the orange lines represents indirect interaction with STAT3 and direct and indirect interaction of the proteins between them; RIGHT: direct and indirect interactions of all the proteins between them without STAT3 (orange lines). A description of the interactome software is given in Materials and Methods.
Similar articles
- Mannose-binding lectin promotes local microvascular thrombosis after transient brain ischemia in mice.
de la Rosa X, Cervera A, Kristoffersen AK, Valdés CP, Varma HM, Justicia C, Durduran T, Chamorro Á, Planas AM. de la Rosa X, et al. Stroke. 2014 May;45(5):1453-9. doi: 10.1161/STROKEAHA.113.004111. Epub 2014 Mar 27. Stroke. 2014. PMID: 24676774 - SMND-309, a novel derivative of salvianolic acid B, protects rat brains ischemia and reperfusion injury by targeting the JAK2/STAT3 pathway.
Zhu H, Zou L, Tian J, Du G, Gao Y. Zhu H, et al. Eur J Pharmacol. 2013 Aug 15;714(1-3):23-31. doi: 10.1016/j.ejphar.2013.05.043. Epub 2013 Jun 11. Eur J Pharmacol. 2013. PMID: 23764464 - Proteomic Analysis of Rat Cerebral Cortex in the Subacute to Long-Term Phases of Focal Cerebral Ischemia-Reperfusion Injury.
Wen M, Jin Y, Zhang H, Sun X, Kuai Y, Tan W. Wen M, et al. J Proteome Res. 2019 Aug 2;18(8):3099-3118. doi: 10.1021/acs.jproteome.9b00220. Epub 2019 Jul 2. J Proteome Res. 2019. PMID: 31265301 - Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion.
Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, Granger DN. Terao S, et al. Stroke. 2008 Sep;39(9):2560-70. doi: 10.1161/STROKEAHA.107.513150. Epub 2008 Jul 17. Stroke. 2008. PMID: 18635850 Free PMC article. - Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection.
Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH. Jung JE, et al. Mol Neurobiol. 2010 Jun;41(2-3):172-9. doi: 10.1007/s12035-010-8102-z. Epub 2010 Feb 17. Mol Neurobiol. 2010. PMID: 20157789 Free PMC article. Review.
Cited by
- STAT3 protects dopaminergic neurons against degeneration in animal model of Parkinson's disease.
Smit RD, Ghosh B, Campion TJ 3rd, Stingel R, Lavell E, Hooper R, Fan X, Soboloff J, Smith GM. Smit RD, et al. Brain Res. 2024 Feb 1;1824:148691. doi: 10.1016/j.brainres.2023.148691. Epub 2023 Nov 27. Brain Res. 2024. PMID: 38030102 - Mitofusin-2 mediates cannabidiol-induced neuroprotection against cerebral ischemia in rats.
Xu BT, Li MF, Chen KC, Li X, Cai NB, Xu JP, Wang HT. Xu BT, et al. Acta Pharmacol Sin. 2023 Mar;44(3):499-512. doi: 10.1038/s41401-022-01004-3. Epub 2022 Oct 13. Acta Pharmacol Sin. 2023. PMID: 36229600 Free PMC article. - New Strategies for Stroke Therapy: Nanoencapsulated Neuroglobin.
Blanco S, Martínez-Lara E, Siles E, Peinado MÁ. Blanco S, et al. Pharmaceutics. 2022 Aug 19;14(8):1737. doi: 10.3390/pharmaceutics14081737. Pharmaceutics. 2022. PMID: 36015363 Free PMC article. Review. - Stroke Proteomics: From Discovery to Diagnostic and Therapeutic Applications.
Hochrainer K, Yang W. Hochrainer K, et al. Circ Res. 2022 Apr 15;130(8):1145-1166. doi: 10.1161/CIRCRESAHA.122.320110. Epub 2022 Apr 14. Circ Res. 2022. PMID: 35420912 Free PMC article. Review. - Biological Implications of a Stroke Therapy Based in Neuroglobin Hyaluronate Nanoparticles. Neuroprotective Role and Molecular Bases.
Peinado MÁ, Ovelleiro D, Del Moral ML, Hernández R, Martínez-Lara E, Siles E, Pedrajas JR, García-Martín ML, Caro C, Peralta S, Morales ME, Ruiz MA, Blanco S. Peinado MÁ, et al. Int J Mol Sci. 2021 Dec 27;23(1):247. doi: 10.3390/ijms23010247. Int J Mol Sci. 2021. PMID: 35008673 Free PMC article.
References
- Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19:2607–2611. - PubMed
- Alexander-Kaufman K, Harper C. Transketolase: observations in alcohol-related brain damage research. Int J Biochem Cell Biol. 2009;41:717–720. - PubMed
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. discussion 166. - PubMed
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS070837/NS/NINDS NIH HHS/United States
- AG05119/AG/NIA NIH HHS/United States
- R01 NS044313/NS/NINDS NIH HHS/United States
- P01 AG005119/AG/NIA NIH HHS/United States
- P01 AG005119-22/AG/NIA NIH HHS/United States
- R01 NS070837/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous