Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on Hedgehog signaling - PubMed (original) (raw)
Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on Hedgehog signaling
Nadia B Hassounah et al. Clin Cancer Res. 2012.
Abstract
Abnormal Hedgehog (Hh) pathway activity has been reported in many cancers, including basal cell carcinomas, medulloblastomas, rhabdomyosarcomas, glioblastomas, and breast and prostate cancers. For this reason, the Hh pathway is a flourishing area for development of anticancer drugs such as Hh ligand antagonists (e.g., 5E1 and robotnikinin), Smo inhibitors (e.g., GDC-0449 and IPI-926), and Gli transcriptional activity inhibitors (e.g., GANT58 and GANT61). It is now clear that primary cilia are required for activation of the Hh pathway in normal vertebrate cells. It is in the primary cilium that both positive and negative effectors of the Hh pathway are processed by posttranslational modifications. In many cancers, preliminary results suggest that primary cilia are lost. As drugs that inhibit different steps of the Hh pathway are developed, it will be important to consider how these drugs will function in the context of primary cilia in the tumor environment. Here, we discuss why some of the Hh inhibitors may be ineffective if primary cilia are lost on cancer cells. Understanding the relationships between clinical inhibitors of the Hh pathway and the presence or absence of primary cilia may turn out to be critical for targeting these therapeutics to the correct population of patients and improving their efficacy. Further work is needed in this area to maximize the potential of these exciting therapeutic targets.
©2012 AACR.
Conflict of interest statement
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Figures
Figure 1. Regulation of the Hedgehog Pathway by Primary Cilia in Normal Cells
Structure of Primary Cilium: The primary cilium contains microtubule bundles (9 doublets arrayed as a cylindrical structure) that are nucleated from the basal body. The microtubule bundles are enclosed in a ciliary membrane that is continuous, but distinct, from the plasma membrane. At the base of the cilium are transition fibers localized in the transition zone. This transition zone is known to restrict passive diffusion of proteins in and out of the cilium. Kinesin 2 moves the IFT complex and its ‘cargo’ (e.g. Gli, Ptch and Smo) towards the plus-end of microtubules (ciliary tip). Dynein 2 moves the IFT complex and its ‘cargo’ towards the minus-end of microtubules (cell body). Hh Regulation: In the absence of Hh (left side) Gli protein is converted to its repressor form (GliR). Also in the absence of Hh, Ptch1 is localized to the ciliary membrane and Smo is kept out of the cilium. In the presence of Hh (right side) Gli protein levels increase in the cilium and Gli is processed into the activator form (GliA) for transport out of the cilium and into the nucleus where it activates Hh target genes. In the presence of Hh, Ptch1 moves out of the cilium and Smo moves into the cilium where it promotes formation of the activator form of Gli (GliA).
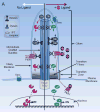
Figure 2. The Role of Cilia in Hedgehog Pathway Activation in Cancer Cells
A. Cancer-Associated overexpression of Hh ligands or mutations in genes such as Ptch1 or Smo, which lie upstream of cilia, will only result in activation of the Hh pathway by increasing GliA levels if cilia are present. If cilia are present, then inhibitors targeting Hh ligand, Smo, and Gli trafficking (grey boxes) will be effective. Inhibitors that target Gli activity downstream of cilia (white box) will also be effective in reducing the Hh pathway in this context. B. Cancer-associated overexpression of Gli1 (GliA) in the presence of cilia will result in low levels of Hh pathway activation. In this context, cilia make the repressor form of Gli (GliR) counterbalancing GliA to reduce over activation of the Hh pathway. Mutations in REN(KCTD11) can also result in increased GliA activity. As this activation is downstream of cilia only the downstream Gli targeting inhibitors (white box) are predicted to be effective. C. Cancer-associated overexpression of Hh ligands or mutations in genes such as Ptch or Smo, which lie upstream of cilia, will not activate the Hh pathway in the absence of cilia. D. Cancer-associated mutations downstream of cilia such as overexpression of Gli1 (GliA) or mutations in Ren(KCTD11) have been documented in cancers and will turn on the Hh pathway in the absence of cilia due to high GliA and low GliR. Therefore, only downstream Gli targeting inhibitors (white box) are predicted to be effective in this scenario.
Similar articles
- The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury.
Grzelak CA, Martelotto LG, Sigglekow ND, Patkunanathan B, Ajami K, Calabro SR, Dwyer BJ, Tirnitz-Parker JE, Watkins DN, Warner FJ, Shackel NA, McCaughan GW. Grzelak CA, et al. J Hepatol. 2014 Jan;60(1):143-51. doi: 10.1016/j.jhep.2013.08.012. Epub 2013 Aug 23. J Hepatol. 2014. PMID: 23978713 - Loss of Primary Cilia Drives Switching from Hedgehog to Ras/MAPK Pathway in Resistant Basal Cell Carcinoma.
Kuonen F, Huskey NE, Shankar G, Jaju P, Whitson RJ, Rieger KE, Atwood SX, Sarin KY, Oro AE. Kuonen F, et al. J Invest Dermatol. 2019 Jul;139(7):1439-1448. doi: 10.1016/j.jid.2018.11.035. Epub 2019 Jan 29. J Invest Dermatol. 2019. PMID: 30707899 Free PMC article. - The primary cilium as a Hedgehog signal transduction machine.
Goetz SC, Ocbina PJ, Anderson KV. Goetz SC, et al. Methods Cell Biol. 2009;94:199-222. doi: 10.1016/S0091-679X(08)94010-3. Epub 2009 Dec 23. Methods Cell Biol. 2009. PMID: 20362092 Free PMC article. - Hedgehog pathway and smoothened inhibitors in cancer therapies.
Chahal KK, Parle M, Abagyan R. Chahal KK, et al. Anticancer Drugs. 2018 Jun;29(5):387-401. doi: 10.1097/CAD.0000000000000609. Anticancer Drugs. 2018. PMID: 29537987 Review. - The primary cilium at the crossroads of mammalian hedgehog signaling.
Wong SY, Reiter JF. Wong SY, et al. Curr Top Dev Biol. 2008;85:225-60. doi: 10.1016/S0070-2153(08)00809-0. Curr Top Dev Biol. 2008. PMID: 19147008 Free PMC article. Review.
Cited by
- Stabilization of primary cilia reduces abortive cell cycle re-entry to protect injured adult CNS neurons from apoptosis.
Choi BKA, D'Onofrio PM, Shabanzadeh AP, Koeberle PD. Choi BKA, et al. PLoS One. 2019 Aug 1;14(8):e0220056. doi: 10.1371/journal.pone.0220056. eCollection 2019. PLoS One. 2019. PMID: 31369591 Free PMC article. - HaCaT cells as a model system to study primary cilia in keratinocytes.
Blanchard G, Pich C, Hohl D. Blanchard G, et al. Exp Dermatol. 2022 Aug;31(8):1276-1280. doi: 10.1111/exd.14626. Epub 2022 Jun 23. Exp Dermatol. 2022. PMID: 35708968 Free PMC article. - Primary cilia and their role in cancer.
Higgins M, Obaidi I, McMorrow T. Higgins M, et al. Oncol Lett. 2019 Mar;17(3):3041-3047. doi: 10.3892/ol.2019.9942. Epub 2019 Jan 17. Oncol Lett. 2019. PMID: 30867732 Free PMC article. Review. - Triptolide inhibits epithelial ovarian tumor growth by blocking the hedgehog/Gli pathway.
Hu L, Gao M, Jiang H, Zhuang L, Jiang Y, Xie S, Zhang H, Wang Q, Chen Q. Hu L, et al. Aging (Albany NY). 2023 Oct 17;15(20):11131-11151. doi: 10.18632/aging.205110. Epub 2023 Oct 17. Aging (Albany NY). 2023. PMID: 37851362 Free PMC article. - Oncoprotein CIP2A promotes the disassembly of primary cilia and inhibits glycolytic metabolism.
Jeong AL, Ka HI, Han S, Lee S, Lee EW, Soh SJ, Joo HJ, Sumiyasuren B, Park JY, Lim JS, Park JH, Lee MS, Yang Y. Jeong AL, et al. EMBO Rep. 2018 May;19(5):e45144. doi: 10.15252/embr.201745144. Epub 2018 Feb 28. EMBO Rep. 2018. PMID: 29491003 Free PMC article.
References
- Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, et al. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics. 2002;1:451–65. - PubMed
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–52. - PubMed
- Smith JC, Northey JG, Garg J, Pearlman RE, Siu KW. Robust method for proteome analysis by MS/MS using an entire translated genome: demonstration on the ciliome of Tetrahymena thermophila. J Proteome Res. 2005;4:909–19. - PubMed
- Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38:961–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA023074/CA/NCI NIH HHS/United States
- P30CA023074/CA/NCI NIH HHS/United States
- R00 HD056965/HD/NICHD NIH HHS/United States
- T32 CA009213/CA/NCI NIH HHS/United States
- R00HD056965/HD/NICHD NIH HHS/United States
- T32CA009213/CA/NCI NIH HHS/United States
- R00 HD056965-05/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous