Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine - PubMed (original) (raw)
Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine
Jeremiah R McDole et al. Nature. 2012.
Abstract
The intestinal immune system is exposed to a mixture of foreign antigens from diet, commensal flora and potential pathogens. Understanding how pathogen-specific immunity is elicited while avoiding inappropriate responses to the background of innocuous antigens is essential for understanding and treating intestinal infections and inflammatory diseases. The ingestion of protein antigen can induce oral tolerance, which is mediated in part by a subset of intestinal dendritic cells (DCs) that promote the development of regulatory T cells. The lamina propria (LP) underlies the expansive single-cell absorptive villous epithelium and contains a large population of DCs (CD11c(+) CD11b(+) MHCII(+) cells) comprised of two predominant subsets: CD103(+) CX(3)CR1(-) DCs, which promote IgA production, imprint gut homing on lymphocytes and induce the development of regulatory T cells, and CD103(-) CX(3)CR1(+) DCs (with features of macrophages), which promote tumour necrosis factor-α (TNF-α) production, colitis, and the development of T(H)17 T cells. However, the mechanisms by which different intestinal LP-DC subsets capture luminal antigens in vivo remains largely unexplored. Using a minimally disruptive in vivo imaging approach we show that in the steady state, small intestine goblet cells (GCs) function as passages delivering low molecular weight soluble antigens from the intestinal lumen to underlying CD103(+) LP-DCs. The preferential delivery of antigens to DCs with tolerogenic properties implies a key role for this GC function in intestinal immune homeostasis.
© 2012 Macmillan Publishers Limited. All rights reserved
Figures
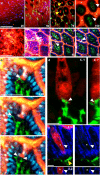
Figure 1. Steady-state trans-epithelial delivery of luminal material in the mouse small intestine
Intestines from CD11cYFP reporter mice injected intraluminally with 10kD dextran (red) imaged with intravital 2P microscopy from the (a) serosal or (b) luminal surfaces. Optical sections at increasing depths (shown in μm) revealed dextran in the lumen (asterisks), the crypts (yellow arrows), on the epithelial surface (unfilled arrow) and columns of dextran that traversed the epithelium (white arrows). Dextran was generally excluded from the LP as identified by CD11cYFP+ LP-DCs (green) below the DAPI stained epithelial nuclei (blue in b). Scale bars = 100μm. (c) Time-lapse recording of LP-DC (green) making repeated contacts with a dextran column (red, white arrow) crossing the epithelium (DAPI stained nuclei, blue). Scale bar=50μm, time stamp=min:sec elapsed time from the start of imaging. (d) Rendered confocal image of a CD11c-YFP+ DC (green) in contact with a dextran filled epithelial cell (red). Panels show orthogonal views; contact is indicated by the white arrow. Scale bar = 5μm. (e) Confocal image of a dextran containing cell (red) bordered by a continuous e-cadherin (blue) positive surface (white arrow). Asterisk indicates the position of an optical section near the cell's center showing intracellular dextran. A CD11cYFP+ DC (green, yellow arrow) positioned near the base of the epithelium. Orthogonal projection (bottom panels); red channel removed (right panels). Scale bar= 5 μm.
Figure 2. GCs are associated with the trans-epithelial passage of luminal material
(a) PAS staining of GCs and (b) dextran columns visualized by 2P microscopy displayed similar morphology and (c) dimensions. (d) Dextran columns were often associated with a nucleus (white arrow). Dextran columns co-localized with GC markers (e) cytokeratin 18 (cyt18, white) and (f) MUC2. (g) Cytokeratin 18 positive cells (white arrows) did not co-localize with the M-cell marker GP2 (yellow arrows). Dextran columns were present in (h) healthy human small intestine and (i) stained positive for cytokeratin 18. Scale bars = 30μm (a, b, and h), 20 μm (d, e, g, and i), and 10μm (f). Error bars = SD.
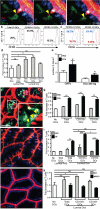
Figure 3. GAPs deliver soluble antigen to CD103+ LP-DCs in the steady-state
Time-lapse 2P imaging of model antigens (red) (a) dextran and (b) bovine serum albumin (BSA) delivered by GAPs to LP-DCs (CD11cYFP = green). Antigen from GAPs co-localized with LP-DCs (yellow arrow) over time). (c) 2P Imaging of CD11cYFP CX3CR1GFP mice. GAPs delivered antigen preferentially to CD103+ LP-DCs (CD11cYFP+ CX3CR1GFP -; green) over CD103− LP-DCs (CD11cYFP+ CX3CR1GFP+; cyan) LP-DCs. (d) Enumeration of GAPs and GAPs delivering antigen to LP-DC subtypes in CD11cYFP CX3CR1GFP mice in 2P recordings. (e) Flow cytometry of LP cells revealed that CD103+ DCs captured more luminal dextran than CD103− DCs. (f) Cytospins on sorted LP-DCs stained with DAPI (blue) and the GC marker cytokeratin 18 (red). (g) Significantly more CD103+ LP-DCs than CD103− LP-DCs stained cytokeratin 18 positive per high-powered field (p= <0.001). (h) Neither DC population expressed detectable cytokeratin 18 mRNA. Scale bar = 15 μm (a and b), 50μm (c), 25μm (f). Data in (g) taken from 9 or more high powered fields (representing >150 cells) Data in (h) performed in triplicate and is representative of two experiments. Error bars = SEM.
Figure 4. GAPs are a source of luminal antigen for CD103+ LP-DCs in the steady-state
(a) 2P time-lapse images of a LP-DC (CD11cYFP+, green) acquiring fluorescent Ova (white arrow) from a GAP (red) in the intestinal epithelium (DAPI stained nuclei, blue). Scale bar = 15 μm; time stamp = min:sec of elapsed time. (b) LP-DC antigen presentation capacity in mice given luminal Ova or PBS assessed on day 3 by (b and c) CFDA dilution and (d) by counting the number of T cells after co-culture with LP-DCs. (c) CD103− LP-DCs induced comparable T cell proliferation to CD103+ LP-DCs when exogenous antigen was added to the in vitro cultures (blue histogram), PBS controls (red histograms). (e and f) 2P imaging of GAPs and LP-DCs in carbamylcholine (CCh) treated mice. Carbamylcholine (CCh) increased the number of GAPs and the co-localization of 10kD dextran (red) with LP-DCs (green). (f, Inset), LP-DCs capturing dextran (white arrowheads). (g) Luminal antigen presentation by LP-DCs following CCh administration as compared to controls given luminal Ova. (h) GF mice had increased GAPs (red = 10kD dextran; DAPI, blue). (i) Luminal antigen presentation by LP-DCs from GF mice was significantly enhanced compared to LP-DCs from SPF housed mice. CD103+ LP-DCs presented luminal antigen significantly better than CD103− LP-DCs from GF mice. (j) 2P imaging of intestines in Math1fl/flVilCre mice (M1KO). M1KO mice lacked GAPs (red, 10 kD dextran) in the epithelium (blue, DAPI). (k) Luminal antigen presentation by LP-DCs from M1KO mice was undetectable. Scale bar in a = 20μm and f = 40μm. ns = not significant. * = p<0.05, ** = p<0.01, *** = p<0.001. Error bars = SEM. Data in d, g, and k are representative of three or more independent experiments, data in (i) was representative of two independent experiments. In (e), n = 15 or more images obtained from 3 animals for each condition.
Comment in
- Mind the GAPs: insights into intestinal epithelial barrier maintenance and luminal antigen delivery.
Miller MJ, Knoop KA, Newberry RD. Miller MJ, et al. Mucosal Immunol. 2014 May;7(3):452-4. doi: 10.1038/mi.2014.4. Epub 2014 Jan 29. Mucosal Immunol. 2014. PMID: 24472846 No abstract available.
Similar articles
- A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity.
Fujimoto K, Karuppuchamy T, Takemura N, Shimohigoshi M, Machida T, Haseda Y, Aoshi T, Ishii KJ, Akira S, Uematsu S. Fujimoto K, et al. J Immunol. 2011 Jun 1;186(11):6287-95. doi: 10.4049/jimmunol.1004036. Epub 2011 Apr 27. J Immunol. 2011. PMID: 21525388 - Epithelial expression of the cytosolic retinoid chaperone cellular retinol binding protein II is essential for in vivo imprinting of local gut dendritic cells by lumenal retinoids.
McDonald KG, Leach MR, Brooke KWM, Wang C, Wheeler LW, Hanly EK, Rowley CW, Levin MS, Wagner M, Li E, Newberry RD. McDonald KG, et al. Am J Pathol. 2012 Mar;180(3):984-997. doi: 10.1016/j.ajpath.2011.11.009. Epub 2012 Jan 2. Am J Pathol. 2012. PMID: 22222225 Free PMC article. - Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism.
Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyártó BZ, Kaplan DH. Welty NE, et al. J Exp Med. 2013 Sep 23;210(10):2011-24. doi: 10.1084/jem.20130728. Epub 2013 Sep 9. J Exp Med. 2013. PMID: 24019552 Free PMC article. - Intestinal CD103+ dendritic cells: master regulators of tolerance?
Scott CL, Aumeunier AM, Mowat AM. Scott CL, et al. Trends Immunol. 2011 Sep;32(9):412-9. doi: 10.1016/j.it.2011.06.003. Epub 2011 Aug 2. Trends Immunol. 2011. PMID: 21816673 Review. - Intestinal dendritic cells in the regulation of mucosal immunity.
Bekiaris V, Persson EK, Agace WW. Bekiaris V, et al. Immunol Rev. 2014 Jul;260(1):86-101. doi: 10.1111/imr.12194. Immunol Rev. 2014. PMID: 24942684 Review.
Cited by
- Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids.
Xie Z, Li M, Qian M, Yang Z, Han X. Xie Z, et al. Nutrients. 2022 Oct 25;14(21):4475. doi: 10.3390/nu14214475. Nutrients. 2022. PMID: 36364738 Free PMC article. - Live Intravital Intestine with Blood Flow Visualization in Neonatal Mice Using Two-photon Laser Scanning Microscopy.
Koike Y, Li B, Chen Y, Ganji N, Alganabi M, Miyake H, Lee C, Hock A, Wu R, Uchida K, Inoue M, Delgado-Olguin P, Pierro A. Koike Y, et al. Bio Protoc. 2021 Mar 5;11(5):e3937. doi: 10.21769/BioProtoc.3937. eCollection 2021 Mar 5. Bio Protoc. 2021. PMID: 33796611 Free PMC article. - pTDP-43 aggregates accumulate in non-central nervous system tissues prior to symptom onset in amyotrophic lateral sclerosis: a case series linking archival surgical biopsies with clinical phenotypic data.
Pattle SB, O'Shaughnessy J, Kantelberg O, Rifai OM, Pate J, Nellany K, Hays N, Arends MJ, Horrocks MH, Waldron FM, Gregory JM. Pattle SB, et al. J Pathol Clin Res. 2023 Jan;9(1):44-55. doi: 10.1002/cjp2.297. Epub 2022 Oct 13. J Pathol Clin Res. 2023. PMID: 36226890 Free PMC article. - β8 Integrin Expression and Activation of TGF-β by Intestinal Dendritic Cells Are Determined by Both Tissue Microenvironment and Cell Lineage.
Boucard-Jourdin M, Kugler D, Endale Ahanda ML, This S, De Calisto J, Zhang A, Mora JR, Stuart LM, Savill J, Lacy-Hulbert A, Paidassi H. Boucard-Jourdin M, et al. J Immunol. 2016 Sep 1;197(5):1968-78. doi: 10.4049/jimmunol.1600244. Epub 2016 Aug 1. J Immunol. 2016. PMID: 27481847 Free PMC article. - Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses.
Khandelwal P, Blanco-Mezquita T, Emami P, Lee HS, Reyes NJ, Mathew R, Huang R, Saban DR. Khandelwal P, et al. PLoS One. 2013 May 14;8(5):e64193. doi: 10.1371/journal.pone.0064193. Print 2013. PLoS One. 2013. PMID: 23691170 Free PMC article.
References
- Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi:10.1038/ni.1622. - PubMed
- Varol C, et al. Intestinal Lamina Propria Dendritic Cell Subsets Have Different Origin and Functions. Immunity. 2009;31:502–512. doi:10.1016/j.immuni.2009.06.025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK064798/DK/NIDDK NIH HHS/United States
- AI095550/AI/NIAID NIH HHS/United States
- R01 AI077600-04/AI/NIAID NIH HHS/United States
- P30 CA91842/CA/NCI NIH HHS/United States
- R21 AI083538-02/AI/NIAID NIH HHS/United States
- F32 DK085941/DK/NIDDK NIH HHS/United States
- R01 AI077600/AI/NIAID NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R21 AI083538/AI/NIAID NIH HHS/United States
- AI077600/AI/NIAID NIH HHS/United States
- U01 AI095550-01/AI/NIAID NIH HHS/United States
- DK064798/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- U01 AI095550/AI/NIAID NIH HHS/United States
- P30-DK52574/DK/NIDDK NIH HHS/United States
- R01 DK064798-08/DK/NIDDK NIH HHS/United States
- DK085941/DK/NIDDK NIH HHS/United States
- AI083538/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous