Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein - PubMed (original) (raw)
Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein
Ari J Firestone et al. Nature. 2012.
Abstract
The conversion of chemical energy into mechanical force by AAA+ (ATPases associated with diverse cellular activities) ATPases is integral to cellular processes, including DNA replication, protein unfolding, cargo transport and membrane fusion. The AAA+ ATPase motor cytoplasmic dynein regulates ciliary trafficking, mitotic spindle formation and organelle transport, and dissecting its precise functions has been challenging because of its rapid timescale of action and the lack of cell-permeable, chemical modulators. Here we describe the discovery of ciliobrevins, the first specific small-molecule antagonists of cytoplasmic dynein. Ciliobrevins perturb protein trafficking within the primary cilium, leading to their malformation and Hedgehog signalling blockade. Ciliobrevins also prevent spindle pole focusing, kinetochore-microtubule attachment, melanosome aggregation and peroxisome motility in cultured cells. We further demonstrate the ability of ciliobrevins to block dynein-dependent microtubule gliding and ATPase activity in vitro. Ciliobrevins therefore will be useful reagents for studying cellular processes that require this microtubule motor and may guide the development of additional AAA+ ATPase superfamily inhibitors.
Figures
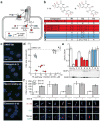
Figure 1. Ciliobrevins disrupt primary cilium-dependent Gli regulation
a, Depiction of Hh signaling with positive (blue) and negative (red) regulators. b, Dihydroquinazolinone structures. c, Ciliary phenotypes after treatment with individual compounds (30 μM) for 24 hours. Scale bar, 10 μm. d, Compound effects on Hh target gene expression in Shh-LIGHT2 cells and cilia size in Shh-EGFP cells. IC50's are the average of three independent experiments ± s.d., and cilia size is defined as the average number of Arl13b-positive pixels per nuclei for ten micrographs ± s.d., each containing approximately 150 cells. Small molecules that block Hh signaling alone (blue) and those that inhibit both Hh pathway activity and ciliogenesis (ciliobrevins; red) are highlighted. e, Gli3FL and Gli3R levels in Shh-EGFP cells treated with Shh-N and either individual dihydroquinazolinones (30 μM), 3 μM cyclopamine, or DMSO for 16 hours. Average Gli3FL/Gli3R ratios for five independent experiments ± s.e.m. and a representative immunoblot are shown, with asterisks indicating p < 0.005 for individual compounds vs. DMSO. f, Ciliary Gli2 levels in Shh-EGFP cells treated with selected ciliobrevins (1 and 5), inactive analogs (2 and 8), or DMSO for 4 hours. Average Gli2 levels in the distal end of at least 25 cilia ± s.e.m. and representative confocal micrographs are shown. Asterisks indicate p < 0.005 for individual compounds vs. DMSO. Scale bar, 1 μm.
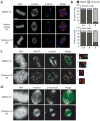
Figure 2. Ciliobrevins disrupt spindle pole assembly and kinetochore-microtubule attachment
a, Mitotic spindles observed in NIH-3T3 cells treated with MG132 for 90 min and subsequently cultured with an inactive analog (2) or ciliobrevin D (5) at a 50-μM dose or DMSO for 1 hour. Staining for DNA, α-tubulin, and γ–tubulin are shown. b, Quantification of spindle phenotypes in NIH-3T3 and HeLa cells treated as described above and scored for either bipolar or abnormal morphologies. Data are the average of three independent experiments ± s.e.m., each including at least 150 spindles. c, Kinetochore-microtubule interactions analyzed in metaphase-arrested NIH-3T3 cells treated with DMSO or 50 μM 5 and then incubated on ice for 10 min. Staining for DNA, the kinetochore marker CREST, and α-tubulin are shown. Insets highlight individual kinetochore-microtubule attachments or untethered kinetochores (400% magnification). d, Localization of p150-Glued in metaphase-arrested NIH-3T3 cells treated with DMSO or 50 μM 5. Staining for DNA, α-tubulin, and p150-Glued are shown. Scale bars: a, 4 μm; c-d, 5 μm.
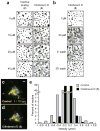
Figure 3. Ciliobrevins inhibit melanosome aggregation and peroxisome motility
a, Brightfield images of Xenopus melanophores treated with various concentrations of ciliobrevin D (5) or an inactive derivative (2) for 10 min, stimulated with melatonin for 30 min, and then paraformaldehyde-fixed. b, Melanophores treated with 5 as before, washed in medium containing melatonin alone, and then imaged. Scale bars, 100 μm. c-d, Motility of GFP-labeled peroxisomes in Drosophila S2 cells cultured in the absence (c) or presence (d) of 5. Overlays of videomicroscopy frames at t = 0 seconds (red) and t = 10 seconds (green) are shown. Scale bar, 5 μm. e, Vector distributions for the GFP-labeled peroxisomes with movement toward and away from the cell center denoted by negative and positive bin values, respectively.
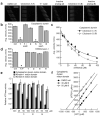
Figure 4. Ciliobrevins inhibit cytoplasmic dynein-dependent microtubule gliding and ATPase activity
a, Kymographs of fluorescent microtubules moving on bovine dynein-coated glass slides in the presence of ATP and either DMSO, ciliobrevin A (1), ciliobrevin D (5), or non-cilia-disrupting analogs (2 and 8). All compounds were tested at a 100-μM concentration, and each vertical frame represents 2 seconds. Scale bar, 10 μm. b, Quantification of the compounds' effects on dynein-dependent microtubule gliding. Data are the average velocities for at least 56 microtubules ± s.e.m. Asterisks indicate p < 10−6 and at least 30% inhibition for individual compounds vs. DMSO. c, Dose responses of 1 and 5 in the dynein-dependent assay. Data are the average velocities for at least 45 microtubules ± s.e.m. d, Effects of 1, 2, 5, 8, and the competitive ATP antagonist adenylyl imidodiphosphate (AMP-PNP) on microtubule gliding driven by the K560 N-terminal fragment of kinesin-1. Dihydroquinazolinones and AMP-PNP were tested at a 100-μM and 1-mM concentrations, respectively. Data are the average velocities for at least 34 microtubules ± s.e.m., and asterisks indicate p < 0.01 for individual compounds vs. DMSO. e, Effects of 1, 2, and 5 on the ATPase activities of recombinant motor domains derived from rat cytoplasmic dynein, human kinesin-1, and human kinesin-5. Compound concentrations in micromolar units are shown, and data are the average ATPase activities for two independent experiments ± s.d. f, Hanes-Woolf plot of rat dynein motor ATPase kinetics demonstrating the nucleotide-competitive activity of 5.
Similar articles
- Cytoplasmic Dynein Antagonists with Improved Potency and Isoform Selectivity.
See SK, Hoogendoorn S, Chung AH, Ye F, Steinman JB, Sakata-Kato T, Miller RM, Cupido T, Zalyte R, Carter AP, Nachury MV, Kapoor TM, Chen JK. See SK, et al. ACS Chem Biol. 2016 Jan 15;11(1):53-60. doi: 10.1021/acschembio.5b00895. Epub 2015 Nov 11. ACS Chem Biol. 2016. PMID: 26555042 Free PMC article. - Chemical structure-guided design of dynapyrazoles, cell-permeable dynein inhibitors with a unique mode of action.
Steinman JB, Santarossa CC, Miller RM, Yu LS, Serpinskaya AS, Furukawa H, Morimoto S, Tanaka Y, Nishitani M, Asano M, Zalyte R, Ondrus AE, Johnson AG, Ye F, Nachury MV, Fukase Y, Aso K, Foley MA, Gelfand VI, Chen JK, Carter AP, Kapoor TM. Steinman JB, et al. Elife. 2017 May 19;6:e25174. doi: 10.7554/eLife.25174. Elife. 2017. PMID: 28524820 Free PMC article. - Dynarrestin, a Novel Inhibitor of Cytoplasmic Dynein.
Höing S, Yeh TY, Baumann M, Martinez NE, Habenberger P, Kremer L, Drexler HCA, Küchler P, Reinhardt P, Choidas A, Zischinsky ML, Zischinsky G, Nandini S, Ledray AP, Ketcham SA, Reinhardt L, Abo-Rady M, Glatza M, King SJ, Nussbaumer P, Ziegler S, Klebl B, Schroer TA, Schöler HR, Waldmann H, Sterneckert J. Höing S, et al. Cell Chem Biol. 2018 Apr 19;25(4):357-369.e6. doi: 10.1016/j.chembiol.2017.12.014. Epub 2018 Jan 27. Cell Chem Biol. 2018. PMID: 29396292 Free PMC article. - Ciliobrevins as tools for studying dynein motor function.
Roossien DH, Miller KE, Gallo G. Roossien DH, et al. Front Cell Neurosci. 2015 Jul 6;9:252. doi: 10.3389/fncel.2015.00252. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26217180 Free PMC article. Review. - Activation of the motor protein upon attachment: Anchors weigh in on cytoplasmic dynein regulation.
Ananthanarayanan V. Ananthanarayanan V. Bioessays. 2016 Jun;38(6):514-25. doi: 10.1002/bies.201600002. Epub 2016 May 3. Bioessays. 2016. PMID: 27143631 Review.
Cited by
- Islet primary cilia motility controls insulin secretion.
Cho JH, Li ZA, Zhu L, Muegge BD, Roseman HF, Lee EY, Utterback T, Woodhams LG, Bayly PV, Hughes JW. Cho JH, et al. Sci Adv. 2022 Sep 23;8(38):eabq8486. doi: 10.1126/sciadv.abq8486. Epub 2022 Sep 23. Sci Adv. 2022. PMID: 36149960 Free PMC article. - Multidimensional screening of pancreatic cancer spheroids reveals vulnerabilities in mitotic and cell-matrix adhesion signaling that associate with metastatic progression and decreased patient survival.
Aquino AF, Runa F, Shoma JF, Todd A, Wallace M, de Barros NR, Kelber JA. Aquino AF, et al. Biochem Biophys Res Commun. 2024 Apr 9;703:149575. doi: 10.1016/j.bbrc.2024.149575. Epub 2024 Feb 6. Biochem Biophys Res Commun. 2024. PMID: 38382357 Free PMC article. - Primary cilia contribute to the aggressiveness of atypical teratoid/rhabdoid tumors.
Blümel L, Qin N, Berlandi J, Paisana E, Cascão R, Custódia C, Pauck D, Picard D, Langini M, Stühler K, Meyer FD, Göbbels S, Malzkorn B, Liebau MC, Barata JT, Jeibmann A, Kerl K, Erkek S, Kool M, Pfister SM, Johann PD, Frühwald MC, Borkhardt A, Reifenberger G, Faria CC, Fischer U, Hasselblatt M, Bartl J, Remke M. Blümel L, et al. Cell Death Dis. 2022 Sep 20;13(9):806. doi: 10.1038/s41419-022-05243-4. Cell Death Dis. 2022. PMID: 36127323 Free PMC article. - KIF3A binds to β-arrestin for suppressing Wnt/β-catenin signalling independently of primary cilia in lung cancer.
Kim M, Suh YA, Oh JH, Lee BR, Kim J, Jang SJ. Kim M, et al. Sci Rep. 2016 Sep 6;6:32770. doi: 10.1038/srep32770. Sci Rep. 2016. PMID: 27596264 Free PMC article. - Impaired autophagic flux and dedifferentiation in podocytes lacking Asah1 gene: Role of lysosomal TRPML1 channel.
Li G, Huang D, Zou Y, Kidd J, Gehr TWB, Li N, Ritter JK, Li PL. Li G, et al. Biochim Biophys Acta Mol Cell Res. 2023 Jan;1870(1):119386. doi: 10.1016/j.bbamcr.2022.119386. Epub 2022 Oct 24. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36302466 Free PMC article.
References
- White SR, Lauring B. AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic. 2007;8:1657–1667. - PubMed
- Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. - PubMed
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM052111/GM/NIGMS NIH HHS/United States
- R01 GM71772/GM/NIGMS NIH HHS/United States
- R24 GM071772/GM/NIGMS NIH HHS/United States
- R01 CA136574/CA/NCI NIH HHS/United States
- R01 GM065933/GM/NIGMS NIH HHS/United States
- R01 GM52111/GM/NIGMS NIH HHS/United States
- R01 GM65933/GM/NIGMS NIH HHS/United States
- R01 GM038839/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources