Glucose regulated protein 78 diminishes α-synuclein neurotoxicity in a rat model of Parkinson disease - PubMed (original) (raw)
Glucose regulated protein 78 diminishes α-synuclein neurotoxicity in a rat model of Parkinson disease
Marina S Gorbatyuk et al. Mol Ther. 2012 Jul.
Abstract
Accumulation of human wild-type (wt) α-synuclein (α-syn) induces neurodegeneration in humans and in experimental rodent models of Parkinson disease (PD). It also leads to endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR). We overexpressed glucose regulated protein 78, also known as BiP (GRP78/BiP), to test the hypothesis that this ER chaperone modulates the UPR, blocks apoptosis, and promotes the survival of nigral dopamine (DA) neurons in a rat model of PD induced by elevated level of human α-syn. We determined that α-syn activates ER stress mediators associated with pancreatic ER kinase-like ER kinase (PERK) and activating transcription factor-6 (ATF6) signaling pathways as well as proaoptotic CCAAT/-enhancer-binding protein homologous protein (CHOP) in nigral DA neurons. At the same time, overexpression of GRP78/BiP diminished α-syn neurotoxicity by down regulating ER stress mediators and the level of apoptosis, promoted survival of nigral tyrosine hydroxylase (TH) positive cells and resulted in higher levels of striatal DA, while eliminating amphetamine induced behavioral asymmetry. We also detected a complex between GRP78/BiP and α-syn that may contribute to prevention of the neurotoxicity caused by α-syn. Our data suggest that the molecular chaperone GRP78/BiP plays a neuroprotective role in α-syn-induced Parkinson-like neurodegeneration.
Figures
Figure 1
The expression of the human wild-type (wt) α-synuclein (α-syn) in the substantia nigra pars compacta (SNc) in 4 weeks after recombinant adeno-associated virus (rAAV) injection. (a) Photomicrographs showing the expression of the human wt α-syn transgene on the injected side of rat brain. α-Syn (red) was expressed in the majority of the tyrosine hydroxylase (TH)-positive neurons (green) in SNc and also can be seen in cells of the mesenphalic tegmentum and in the SN pars reticulate. Bar = 0.5 mm. (b) Measurement of α-syn and TH expression in animals that had been injected with human wt α-syn at 4 weeks postinjection. Samples of nigral extracts taken from the uninjected left side (L) and injected right side (R) of individual animals (462-465) were electrophoresed on acrylamide gels. Fifty micrograms of tissue extract was analyzed by immunoblots using antibodies that recognize both human and rat α-syn, as well as TH or GAPDH antibodies. The amount of TH enzyme was normalized to GAPDH and the ratio of the injected versus uninjected sides was calculated. The ratio of α-syn on the injected and uninjected sides was calculated for each animal using the same approach.
Figure 2
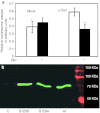
Immunoblot analysis of the unfolded protein response (UPR) markers in nigral tissue at 4 weeks after recombinant adeno-associated virus (rAAV) human wild-type (wt) α-synuclein (α-syn) and rAAV green fluorescent protein (GFP) injections. Overexpression of human α-syn in the substantia nigra pars compacta (SNc) at 4 weeks postinjection leads to a significant upregulation of peIF2α, ATF4, and pATF6 (50 kDa) proteins. The activation of these UPR mediators results a significant overexpression of the proapoptotic C/EBP homologous protein (CHOP) protein. Tukey's post hoc results are indicated as *, **, ***P < 0.05, 0.01, and 0.001, respectively versus α-syn; n = 4–6 for each group.
Figure 3
Human wild-type (wt) α-synuclein (α-syn) and GRP78/BiP cotransfection of SH-SY5Y cells. (a) GRP78/BiP overexpression protects SH-SY5Y cells from apoptosis induced by human wt α-syn. Apoptotic signal was measured in 48 hours by nucleosome release ELISA assay. Unpaired _t_-test is indicated as **P < 0.01; n = 4. (b) Overexpressed BiP binds to the human wt α-syn. Detection of BiP-Flag (green band) by anti-Flag Ab in a protein extract from SH-SY5Y coexpressing BiP-Flag and human wt α-syn was performed by immunoprecipitation with the human α-syn-specific antibody. Western blot analysis detected the band corresponding to 78 kDa by Odyssey Infrared System. The two additional mutants shown (S129A and S129D), while not related to this study, demonstrate that GRP78 can interact with both the phosphorylated (S129D) and nonphosphorylated forms of wt α-syn. As a negative control (C) for the precipitation study mouse anti-green fluorescent protein (GFP) antibody was used instead of human wt α-syn antibody.
Figure 4
Expression of BiP-Flag and human α-synuclein (α-syn) in rat substantia nigra pars compacta (SNc). (a) Images of the SNc expressing BiP-Flag protein alone on a side of injection. Left side (top image) was intact and did not demonstrate staining with Flag-specific antibodies (bar = 150 µm). In red—detection of Flag-tag with Cy3-conjugated secondary Ab, in green—detection of the vesicle monoamine transporter-2 (VMAT) with Cy2-conjugated secondary Ab. Higher resolution images (bottom) show BiP-Flag expression in most if not all nigral dopamine (DA) neurons (bar = 25 µm). (b) Coexpression of both BiP-Flag and human wild-type (wt) α-syn was found in SNc of rats injected with both viruses (bar = 25 µm). (c) Coexpression of BiP-Flag and human α-syn do not inhibit an expression level of each other. Expression level of BiP-Flag or human α-syn in a dual injection is equivalent to one with a single viral injection. Western blot (WB) visualized with an antibody specific to Flag and human α-syn. “Cont” is noninjected side of single rat taken as a control. (d) Nigral tissue from 4 weeks animals was extracted and pooled (n = 4) and then immunoblotted with antibody to the GRP78/BiP. A loading control (tubulin) is also shown. Extracts from the uninjected side (L) were compared with the injected side (R). Expression of total GRP78/BiP protein detected with anti-GRP78/BiP antibody was about 39% higher in injected sides compared to uninjected. (e) Image of immunoblot of immunoprecipitated protein extract isolated from the SNc injected with a viral combination. Immunoprecipitation was done using anti-Flag antibody. The membrane from the immunoblot was treated with a specific antibody against human α-syn to detect the band corresponding to 19 kDa α-syn. As a negative control for the precipitation study mouse immunoglobulin was used instead of anti-Flag antibody (data not shown).
Figure 5
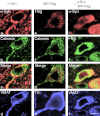
Confocal microscopy of representative cells in substantia nigra pars compacta (SNc) of rats at 4 weeks after injections of (a–d) recombinant adeno-associated virus (rAAV) α-synuclein (α-syn) alone, (e–h) rAAV BiP-Flag alone, or (i–l) both α-syn and BiP-Flag vectors. (a–d) SNc neuron expressing α-syn alone detected with antibody specific to human wild-type (wt) α-syn (red). The image shows localization of accumulating α-syn mostly along axonal and cell body membrane, and to lesser extend in cytosol (a). Immunocytochemical detection of endoplasmic reticulum (ER) marker, calnexin (green) (b) revealed that α-syn might accumulate partially on the ER (c). Vesicle monoamine transporter-2 (VMAT) immunostaining (blue) of the same cell confirms that colocalization was identified in nigral dopamine (DA) neuron (d). (e–h) Overexpression of BiP-Flag alone in SNc neuron detected with anti-Flag antibody (red). Immunostaining of the same cell for calnexin (green) (f) allowed for colocalization with the Flag antibody, as seen on merged image (g), primarily on ER (yellow). However, some dust like red staining can be also found outside of the ER suggesting that overexpressed BiP might escape ER recruitment. Immunostaining for tyrosine hydroxylase (TH) (blue) in the same cell was used as a marker of DA producing neuron in the SNc (h). (i–l) Nigral neuron expressing both human wt α-syn (red) (i) and BiP-Flag (green) (j). Note that α-syn immunoreactivity spreads diffusely (i) compared to overexpression of human α-syn alone (a). Bar = 5 µm.
Figure 6
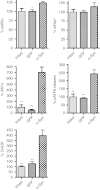
Overexpression of GRP78/BiP protein in the substantia nigra pars compacta (SNc) of Parkinson disease (PD) rats leads to reprogramming the endoplasmic reticulum (ER) stress signaling. At 4 weeks of PD progression GRP78/BiP significantly reduced accumulation of the ER stress hallmarks such as cleaved pATF6 (pATF6 50) and ATF4 (PERK pathway). Production of C/EBP homologous protein (CHOP) protein was also down regulated at 4 and 8 weeks in rat SNc injected with both α-synuclein (α-syn) and GRP78/BiP compared to SNc of rats injected with α-syn. Injection with recombinant adeno-associated virus (rAAV) BiP was not a statistically different from green fluorescent protein (GFP) injection or intact control. Tukey's post hoc results are indicated as *, **, ***P < 0.05, 0.01, and 0.001, respectively versus α-syn; #P < 0.05 versus α-syn + GRP78/BiP; n = 4–7 for each group.
Figure 7
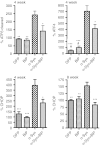
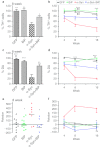
Overexpression of GRP78/BiP protein prevents loss of tyrosine hydroxylase (TH)-positive cells and striatal dopamine (DA) level as well as eliminates behavior deficit. (a) Unbiased estimation of TH+ cells remaining in substantia nigra pars compacta (SNc) on the injected side as a percentage of the uninjected side ± SE at 8 weeks postinjection. A number of TH-positive neurons counted in α-synuclein (α-syn) injected SNc was dramatically reduced compared to control. However, coexpression of α-syn and GRP78/BiP proteins led to significant rescue of TH-positive cells preventing cell death compared to a single α-syn injection. These animals have only a 24% loss of TH-positive cells compared to green fluorescent protein (GFP) which was not statistically significant. GRP78/BiP overexpression alone did not reduce the number of TH-positive cells. One-way ANOVA analysis. Tukey post hoc results are indicated as *,**P < 0.05 and 0.01, respectively versus α-syn; n = 4–11 per group. (b) The graph shows the % TH+ cells remaining at different times postinjection. Two-way ANOVA statistics. Bonferroni post-tests are indicated as *, **, ***P < 0.05, 0.01, and 0.001, respectively versus α-syn; ##P < 0.01 versus α-syn + GRP78/BiP; n = 4–11 for each group at each time point. (c) At 8 weeks, decline of DA in the striatum injected with a virus expressing α-syn was 75% compared to GFP-injected animals. Overexpression of GRP78/BiP protein along with α-syn significantly (by 46%) preserved the dopamine (DA) level of injected animals observed in a single α-syn injection. This also was a statistically reliable from GFP control. Overexpression of GRP78/BiP protein alone did not block the synthesis of DA in injected SNcs compared to control GFP injection. One-way ANOVA analysis. Tukey post hoc results are indicated as **, ***P < 0.01, and 0.001, respectively versus α-syn; #P < 0.05 versus α-syn + GRP78/BiP; n = 5–10 per group. (d) Changes in DA levels of different experimental group at 16 weeks were similar to 8 weeks. Two-way ANOVA statistics. Bonferroni post-tests are indicated as *,**,***P < 0.05, 0.01 and 0.001, respectively versus α-syn; ##P < 0.01 versus α-syn + GRP78/BiP; n = 4–10 for each group at each time point. (e) Amphetamine induced rotation test at 8 weeks revealed a significant number of rotations toward the injected side (positive, ipsilateral rotation) only in α-syn-injected rats compared to GRP78/BiP and α-syn plus GRP78/BiP. Amphetamine induced rotation was measured for 90 minutes and the average rotation plus SE is shown. One-way ANOVA analysis. Tukey's post hoc results are indicated as *P < 0.05 versus α-syn; n = 9–13 per group. (f) Amphetamine rotation versus time postinjection in α-syn-injected rats compared to GFP, GRP78/BiP, and α-syn plus GRP78/BiP. Two-way ANOVA analysis. Bonferroni post-test is indicated as *,**P < 0.05 and 0.01 versus α-syn; n = 6–18 per group.
Similar articles
- Empagliflozin alleviates endoplasmic reticulum stress and augments autophagy in rotenone-induced Parkinson's disease in rats: Targeting the GRP78/PERK/eIF2α/CHOP pathway and miR-211-5p.
Motawi TK, Al-Kady RH, Abdelraouf SM, Senousy MA. Motawi TK, et al. Chem Biol Interact. 2022 Aug 1;362:110002. doi: 10.1016/j.cbi.2022.110002. Epub 2022 May 30. Chem Biol Interact. 2022. PMID: 35654124 - The loss of glucose-regulated protein 78 (GRP78) during normal aging or from siRNA knockdown augments human alpha-synuclein (α-syn) toxicity to rat nigral neurons.
Salganik M, Sergeyev VG, Shinde V, Meyers CA, Gorbatyuk MS, Lin JH, Zolotukhin S, Gorbatyuk OS. Salganik M, et al. Neurobiol Aging. 2015 Jun;36(6):2213-23. doi: 10.1016/j.neurobiolaging.2015.02.018. Epub 2015 Mar 5. Neurobiol Aging. 2015. PMID: 25863526 Free PMC article. - α-Synuclein-mediated inhibition of ATF6 processing into COPII vesicles disrupts UPR signaling in Parkinson's disease.
Credle JJ, Forcelli PA, Delannoy M, Oaks AW, Permaul E, Berry DL, Duka V, Wills J, Sidhu A. Credle JJ, et al. Neurobiol Dis. 2015 Apr;76:112-125. doi: 10.1016/j.nbd.2015.02.005. Epub 2015 Feb 26. Neurobiol Dis. 2015. PMID: 25725420 - Molecular signal networks and regulating mechanisms of the unfolded protein response.
Gong J, Wang XZ, Wang T, Chen JJ, Xie XY, Hu H, Yu F, Liu HL, Jiang XY, Fan HD. Gong J, et al. J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review. - Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.
Perfeito R, Cunha-Oliveira T, Rego AC. Perfeito R, et al. Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
Cited by
- Gene therapy for neurodegenerative disorders: advances, insights and prospects.
Chen W, Hu Y, Ju D. Chen W, et al. Acta Pharm Sin B. 2020 Aug;10(8):1347-1359. doi: 10.1016/j.apsb.2020.01.015. Epub 2020 Jan 31. Acta Pharm Sin B. 2020. PMID: 32963936 Free PMC article. Review. - Advancements in Genetic and Biochemical Insights: Unraveling the Etiopathogenesis of Neurodegeneration in Parkinson's Disease.
Ratan Y, Rajput A, Pareek A, Pareek A, Jain V, Sonia S, Farooqui Z, Kaur R, Singh G. Ratan Y, et al. Biomolecules. 2024 Jan 5;14(1):73. doi: 10.3390/biom14010073. Biomolecules. 2024. PMID: 38254673 Free PMC article. Review. - Parallel mechanisms for direct and indirect membrane protein trafficking by synucleins.
Oaks AW, Sidhu A. Oaks AW, et al. Commun Integr Biol. 2013 Nov 1;6(6):e26794. doi: 10.4161/cib.26794. Epub 2013 Nov 13. Commun Integr Biol. 2013. PMID: 24563712 Free PMC article. Review. - Insights Into the Role of Platelet-Derived Growth Factors: Implications for Parkinson's Disease Pathogenesis and Treatment.
Li D, Huang LT, Zhang CP, Li Q, Wang JH. Li D, et al. Front Aging Neurosci. 2022 Jul 1;14:890509. doi: 10.3389/fnagi.2022.890509. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35847662 Free PMC article. Review. - Endoplasmic reticulum stress: New insights into the pathogenesis and treatment of retinal degenerative diseases.
Gorbatyuk MS, Starr CR, Gorbatyuk OS. Gorbatyuk MS, et al. Prog Retin Eye Res. 2020 Nov;79:100860. doi: 10.1016/j.preteyeres.2020.100860. Epub 2020 Apr 6. Prog Retin Eye Res. 2020. PMID: 32272207 Free PMC article. Review.
References
- Fearnley JM., and, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114 (Pt 5):2283–2301. - PubMed
- McGeer PL, Itagaki S, Akiyama H., and, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988;24:574–576. - PubMed
- Auluck PK, Caraveo G., and, Lindquist S. a-Synuclein: membrane interactions and toxicity in Parkinson's disease. Annu Rev Cell Dev Biol. 2010;26:211–233. - PubMed
- Chu Y., and, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson's disease. Neurobiol Dis. 2007;25:134–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS69574/NS/NINDS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- R01 EY020905/EY/NEI NIH HHS/United States
- R01 NS069574/NS/NINDS NIH HHS/United States
- P01HL59412/HL/NHLBI NIH HHS/United States
- R01EY020905/EY/NEI NIH HHS/United States
- R01 EY020846/EY/NEI NIH HHS/United States
- P01HL51811/HL/NHLBI NIH HHS/United States
- P01 HL059412/HL/NHLBI NIH HHS/United States
- R01EY020846/EY/NEI NIH HHS/United States
- P01 HL051811/HL/NHLBI NIH HHS/United States
- P30 EY021721/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous