Tyrosine kinase signaling and the emergence of multicellularity - PubMed (original) (raw)
Review
Tyrosine kinase signaling and the emergence of multicellularity
W Todd Miller. Biochim Biophys Acta. 2012 Jun.
Abstract
Tyrosine phosphorylation is an essential element of signal transduction in multicellular animals. Although tyrosine kinases were originally regarded as specific to the metazoan lineage, it is now clear that they evolved prior to the split between unicellular and multicellular eukaryotes (≈600million years ago). Genome analyses of choanoflagellates and other protists show an abundance of tyrosine kinases that rivals the most complex animals. Some of these kinases are orthologs of metazoan enzymes (e.g., Src), but others display unique domain compositions not seen in any metazoan. Biochemical experiments have highlighted similarities and differences between the unicellular and multicellular tyrosine kinases. In particular, it appears that the complex systems of kinase autoregulation may have evolved later in the metazoan lineage.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
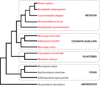
Fig. 1. Distribution of tyrosine kinases
The eukaryotic tree of life is shown schematically. Species containing eukaryotic-like tyrosine kinases are shown in red.
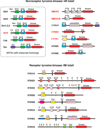
Fig. 2. Tyrosine kinases in Monosiga brevicollis
The domain arrangements of representative nonreceptor tyrosine kinases (NRTKs, top) and receptor tyrosine kinases (RTKs, bottom) are shown. Domain predictions are based on the analysis in ref. [15], and a complete list of kinases is given in the supporting information to that paper. Tyrosine kinase catalytic domains are shown in red. At the top left, all Monosiga brevicollis NRTKs with clear metazoan homologs (based on kinase domain sequence similarity) are boxed. Names given in red are NRTKs with domain combinations that have not been observed in metazoans. List of domain abbreviations: Myr, myristoylation sequence; SH3, Src homology 3; SH2, Src homology 2; C2, phospholipid binding domain; PH, pleckstrin homology; PTB, phosphotyrosine binding domain; pseudokin, predicted inactive kinase domain; CH, calponin homology; FYVE, Fab1/YOTB/Vac1/EEA1 zinc-finger domain; LDL, low density lipoprotein receptor motif; tm, transmembrane sequence; SCR, short consensus repeat/complement control protein domain; E, epidermal growth factor repeat; Cys, cysteine-rich region; HYR, repeats similar to hyaline domains; Cupin, barrel-like fold seen in cupin family proteins; LRR, leucine-rich repeats; Fn3, fibronectin type 3 domain.
Fig. 3. Csk-mediated inhibition of Src
In the active conformation of Src (left), Tyr527 in the C-terminal tail is unphosphorylated, and the SH2 and SH3 domains are disengaged from their intramolecular ligands. The SH3 and SH2 domains bind to ligands on potential substrates, targeting them for phosphorylation by the kinase domain. Phosphorylation of Tyr527 (red circle) produces an intramolecular interaction with the SH2 domain of Src. This interaction, together with an interaction between the SH3 domain and a polyproline type II helix in the linker region, stabilizes the autoinhibited conformation of Src. In the choanoflagellates Monosiga ovata and Monosiga brevicollis, the Csk homologs phosphorylate the residue equivalent to Tyr527, but this does not repress Src activity [16, 29]. The domain structure of Src family kinases and other NRTKs may have arisen initially to fulfill the substrate targeting function [31, 39].
Similar articles
- Signaling properties of a non-metazoan Src kinase and the evolutionary history of Src negative regulation.
Li W, Young SL, King N, Miller WT. Li W, et al. J Biol Chem. 2008 May 30;283(22):15491-501. doi: 10.1074/jbc.M800002200. Epub 2008 Apr 4. J Biol Chem. 2008. PMID: 18390552 Free PMC article. - PTB domain-directed substrate targeting in a tyrosine kinase from the unicellular choanoflagellate Monosiga brevicollis.
Prieto-Echagüe V, Chan PM, Craddock BP, Manser E, Miller WT. Prieto-Echagüe V, et al. PLoS One. 2011 Apr 26;6(4):e19296. doi: 10.1371/journal.pone.0019296. PLoS One. 2011. PMID: 21541291 Free PMC article. - Evolution of the phospho-tyrosine signaling machinery in premetazoan lineages.
Pincus D, Letunic I, Bork P, Lim WA. Pincus D, et al. Proc Natl Acad Sci U S A. 2008 Jul 15;105(28):9680-4. doi: 10.1073/pnas.0803161105. Epub 2008 Jul 3. Proc Natl Acad Sci U S A. 2008. PMID: 18599463 Free PMC article. - Protein tyrosine kinase structure and function.
Hubbard SR, Till JH. Hubbard SR, et al. Annu Rev Biochem. 2000;69:373-98. doi: 10.1146/annurev.biochem.69.1.373. Annu Rev Biochem. 2000. PMID: 10966463 Review. - Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade.
Avruch J, Khokhlatchev A, Kyriakis JM, Luo Z, Tzivion G, Vavvas D, Zhang XF. Avruch J, et al. Recent Prog Horm Res. 2001;56:127-55. doi: 10.1210/rp.56.1.127. Recent Prog Horm Res. 2001. PMID: 11237210 Review.
Cited by
- Complex protein interactions mediate Drosophila Lar function in muscle tissue.
Kawakami J, Brooks D, Zalmai R, Hartson SD, Bouyain S, Geisbrecht ER. Kawakami J, et al. PLoS One. 2022 May 27;17(5):e0269037. doi: 10.1371/journal.pone.0269037. eCollection 2022. PLoS One. 2022. PMID: 35622884 Free PMC article. - Temperature sensitivities of metazoan and pre-metazoan Src kinases.
Miller WT. Miller WT. Biochem Biophys Rep. 2020 Jun 10;23:100775. doi: 10.1016/j.bbrep.2020.100775. eCollection 2020 Sep. Biochem Biophys Rep. 2020. PMID: 32566764 Free PMC article. - Regulation of Src and Csk nonreceptor tyrosine kinases in the filasterean Ministeria vibrans.
Schultheiss KP, Craddock BP, Suga H, Miller WT. Schultheiss KP, et al. Biochemistry. 2014 Mar 4;53(8):1320-9. doi: 10.1021/bi4016499. Epub 2014 Feb 18. Biochemistry. 2014. PMID: 24520931 Free PMC article. - Comparative genomics reveals the origin of fungal hyphae and multicellularity.
Kiss E, Hegedüs B, Virágh M, Varga T, Merényi Z, Kószó T, Bálint B, Prasanna AN, Krizsán K, Kocsubé S, Riquelme M, Takeshita N, Nagy LG. Kiss E, et al. Nat Commun. 2019 Sep 9;10(1):4080. doi: 10.1038/s41467-019-12085-w. Nat Commun. 2019. PMID: 31501435 Free PMC article. - Phosphorylation control of the ubiquitin ligase Cbl is conserved in choanoflagellates.
Amacher JF, Hobbs HT, Cantor AC, Shah L, Rivero MJ, Mulchand SA, Kuriyan J. Amacher JF, et al. Protein Sci. 2018 May;27(5):923-932. doi: 10.1002/pro.3397. Epub 2018 Apr 14. Protein Sci. 2018. PMID: 29498112 Free PMC article.
References
- Manning G, Plowman GD, Hunter T, Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends in biochemical sciences. 2002;27:514–520. - PubMed
- Lee DC, Jia Z. Emerging structural insights into bacterial tyrosine kinases. Trends in biochemical sciences. 2009;34:351–357. - PubMed
- Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. - PubMed
- King N. The unicellular ancestry of animal development. Developmental cell. 2004;7:313–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous