Rhinovirus-induced IL-1β release from bronchial epithelial cells is independent of functional P2X7 - PubMed (original) (raw)
Rhinovirus-induced IL-1β release from bronchial epithelial cells is independent of functional P2X7
Lei Shi et al. Am J Respir Cell Mol Biol. 2012 Sep.
Abstract
Airway epithelial cell defenses to viral infections are often compromised in disease or injury. Danger molecules, including ATP, are released during infection and contribute to nucleotide receptor-dependent inflammatory responses, largely through P2X(7). Although respiratory epithelium has been shown to express a variety of nucleotide receptors, the functional contribution of P2X(7) to the epithelial cell inflammatory response is unclear. We used human donor bronchial epithelial cells (BECs) and primary brushed epithelium to explore responses upon nucleotide and Toll-like receptor stimulation. P2X(7) messenger RNA and protein were observed in unprimed BECs, whereas inflammatory cytokine stimulation increased both messenger RNA and protein. Functional pore activity characteristic of P2X(7) was observed in BECs, and IL-1β was rapidly released by BECs after Toll-like receptor 3 agonist, polyinosine-polycytidylic acid, priming followed by ATP administration, although no change was observed in IL-18 release. BECs produced more IL-1β after stimulation with polyinosine-polycytidylic acid than LPS, showing a different preferential response than monocytes. In addition, blockade of nucleotide receptors with oxidized ATP significantly increased human rhinovirus (HRV) recovered 24 hours after infection in BECs, whereas 2'-3'-O-(4-benzoylbenzoyl) ATP treatment of brushed epithelial cells and respiratory cell lines nonsignificantly decreased HRV recovery. IL-1β release was detected after HRV infection in both BECs and brushed cells, but BzATP did not significantly increase IL-1β release further. BEC processing of pro-IL-1β to the mature, cleaved, 17-kD form was confirmed by Western blotting. These results support the expression of functional P2X(7) in human lung epithelium, although its role in epithelial pathogen defense is likely independent of IL-1 family cytokine processing.
Figures
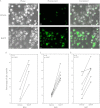
Figure 1.
Bronchial epithelial cells (BECs) have functional P2X7 pore activity. Fluorescent dye, YO-PRO-1, uptake in BECs after 2′-3′-O-(4-benzoylbenzoyl) ATP (BzATP) stimulation was observed by fluorescent microscopy and counted as a proportion of all cells. Representative images of BEC B45 are shown (A). The percentage of adherent BECs containing YO-PRO-1 was calculated and BzATP-treated cells demonstrated a significant increase of uptake for all three BECs (B_–_D). Unpaired t tests were performed within each group. Scale bar, 50 μm.
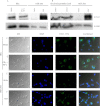
Figure 2.
P2X7 is detected in primary respiratory epithelial cells. BECs cultured with vehicle or TNF-α/IFN-γ for 24 hours (A) and brushed epithelial cells from two donors (B) were probed for P2X7 by Western blotting, and indicate GRB2 (growth factor receptor-bound protein 2) as a loading control. Human embryonic kidney (HEK)-293 cells with vector or P2X7 were used as controls. BECs treated with vehicle (C) or TNF-α/IFN-γ (D) for 48 hours were examined for P2X7 protein. DAPI (4′,6-diamidino-2-phenylindole) and secondary antibody were coincubated before visualization. HEK-293 cells contain an empty vector (E) or express P2X7 (F) for negative and positive controls, respectively. Differential interference contrast and fluorescent signals are combined in the right panels. Scale bar, 10 μm.
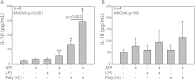
Figure 3.
ATP increases IL-1β release from polyinosine-polycytidylic acid (Poly (I:C))–primed BECs. Supernatants from BEC B45 (n = 4) primed with LPS or Poly (I:C) were collected after stimulation with ATP for 30 minutes and analyzed by ELISA. IL-1β levels were augmented by Poly (I:C) priming, which was further increased with ATP treatment (A). IL-18 did not demonstrate the same pattern of release (B). The limit of detection for both cytokines was 12.5 pg/ml and data are shown as mean ± SD. Pairwise comparisons were Bonferroni corrected. *P < 0.05 compared with vehicle-, ATP-, LPS-, and ATP/LPS-treated cells. **P < 0.05 compared with vehicle.
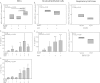
Figure 4.
Nucleotide receptor blockade increases HRV recovery after infection, whereas HRV infection increases IL-1 family cytokine release. Cellular HRV levels at 24 hours were affected by nucleotide receptor modulators. Oxidized ATP (oATP) treatment of BECs increased HRV1a compared with both vehicle and BzATP groups (A), whereas brushed epithelial cells trended to a small decrease with BzATP treatment (B). Respiratory cell lines A549 (C) and BEAS-2B (F) transfected with either vector control or P2X7 were also infected with HRV1a for 24 hours. Supernatants of BECs or brushed epithelial cells treated with oATP and/or BzATP and incubated with HRV1a were analyzed for cytokine production. BEC IL-1β measured at 24 hours (D) was increased with HRV infection in all groups compared with vehicle or oATP treated. Epithelial cells from bronchoscopic brushings showed similar results with IL-1β (E), generating more IL-1β in the supernatant at 24 hours compared with vehicle, but not BzATP-treated cells. BEC IL-18 measured at 24 hours (G) was increased in all HRV groups. Pairwise comparisons are Bonferroni corrected. For cytokine measures: *P < 0.05 compared with vehicle-, BzATP-, and oATP-treated cells; **P < 0.05 compared with vehicle- and oATP-treated cells; ***P < 0.05 compared with vehicle. Box plots display median and interquartile range for A–C and F, and bar graphs show mean ± SD for D, E, and G.
Figure 5.
IL-1β released from BECs is cleaved and demonstrates functional activity. Western blotting of concentrated supernatants from BEC B45 infected for 24 hours with HRV1a and/or stimulated with BzATP is shown with detectable bands similar in size to the positive control 17-kD band from primed THP-1 cells, and is representative of three experiments. Immunoblots with Cell Signaling (Danvers, MA) (A) and Santa Cruz Biotechnology (Santa Cruz, CA) (B) antibodies display preferential detection of the cleaved and pro forms of IL-1β, respectively. Positive control indicates LPS-primed and ATP-treated THP-1 cells. Molecular weight markers are shown on the right. (C) Supernatants from BECs primed with Poly (I:C) or vehicle and treated with ATP were used to culture A549 cells for 24 hours. Increasing concentrations of IL-1 receptor antagonist (IL-1RA) were added and IL-8 was measured by ELISA. *P < 0.05 compared with IL-8 release with no IL-1RA. Data points are the average of technical duplicates.
Similar articles
- Involvement of purinergic receptors and NOD-like receptor-family protein 3-inflammasome pathway in the adenosine triphosphate-induced cytokine release from macrophages.
Gicquel T, Victoni T, Fautrel A, Robert S, Gleonnec F, Guezingar M, Couillin I, Catros V, Boichot E, Lagente V. Gicquel T, et al. Clin Exp Pharmacol Physiol. 2014 Apr;41(4):279-86. doi: 10.1111/1440-1681.12214. Clin Exp Pharmacol Physiol. 2014. PMID: 24472059 - TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo.
He Y, Franchi L, Núñez G. He Y, et al. J Immunol. 2013 Jan 1;190(1):334-9. doi: 10.4049/jimmunol.1202737. Epub 2012 Dec 7. J Immunol. 2013. PMID: 23225887 Free PMC article. - The role of interleukin-1 and interleukin-18 in pro-inflammatory and anti-viral responses to rhinovirus in primary bronchial epithelial cells.
Piper SC, Ferguson J, Kay L, Parker LC, Sabroe I, Sleeman MA, Briend E, Finch DK. Piper SC, et al. PLoS One. 2013 May 28;8(5):e63365. doi: 10.1371/journal.pone.0063365. Print 2013. PLoS One. 2013. PMID: 23723976 Free PMC article. - P2X7 receptor activation amplifies lipopolysaccharide-induced vascular hyporeactivity via interleukin-1 beta release.
Chiao CW, Tostes RC, Webb RC. Chiao CW, et al. J Pharmacol Exp Ther. 2008 Sep;326(3):864-70. doi: 10.1124/jpet.107.135350. Epub 2008 Jun 16. J Pharmacol Exp Ther. 2008. PMID: 18559654 Free PMC article. - Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions.
Lenertz LY, Gavala ML, Zhu Y, Bertics PJ. Lenertz LY, et al. Immunol Res. 2011 May;50(1):22-38. doi: 10.1007/s12026-011-8203-4. Immunol Res. 2011. PMID: 21298493 Free PMC article. Review.
Cited by
- Interaction between allergy and innate immunity: model for eosinophil regulation of epithelial cell interferon expression.
Mathur SK, Fichtinger PS, Kelly JT, Lee WM, Gern JE, Jarjour NN. Mathur SK, et al. Ann Allergy Asthma Immunol. 2013 Jul;111(1):25-31. doi: 10.1016/j.anai.2013.05.010. Ann Allergy Asthma Immunol. 2013. PMID: 23806456 Free PMC article. - Inflammasome signalling pathway in the regulation of inflammation - its involvement in the development and exacerbation of asthma and chronic obstructive pulmonary disease.
Panek I, Liczek M, Gabryelska A, Rakoczy I, Kuna P, Panek M. Panek I, et al. Postepy Dermatol Alergol. 2023 Aug;40(4):487-495. doi: 10.5114/ada.2022.118077. Epub 2022 Jul 12. Postepy Dermatol Alergol. 2023. PMID: 37692274 Free PMC article. Review. - P2X7 signaling influences the production of pro-resolving and pro-inflammatory lipid mediators in alveolar macrophages derived from individuals with asthma.
Townsend EA, Guadarrama A, Shi L, Roti Roti E, Denlinger LC. Townsend EA, et al. Am J Physiol Lung Cell Mol Physiol. 2023 Oct 1;325(4):L399-L410. doi: 10.1152/ajplung.00070.2023. Epub 2023 Aug 15. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37581221 Free PMC article. - IL-1α mediates cellular cross-talk in the airway epithelial mesenchymal trophic unit.
Hill AR, Donaldson JE, Blume C, Smithers N, Tezera L, Tariq K, Dennison P, Rupani H, Edwards MJ, Howarth PH, Grainge C, Davies DE, Swindle EJ. Hill AR, et al. Tissue Barriers. 2016 Jun 28;4(3):e1206378. doi: 10.1080/21688370.2016.1206378. eCollection 2016 Jul-Sep. Tissue Barriers. 2016. PMID: 27583193 Free PMC article. - Inflammasome activation is required for human rhinovirus-induced airway inflammation in naive and allergen-sensitized mice.
Han M, Bentley JK, Rajput C, Lei J, Ishikawa T, Jarman CR, Lee J, Goldsmith AM, Jackson WT, Hoenerhoff MJ, Lewis TC, Hershenson MB. Han M, et al. Mucosal Immunol. 2019 Jul;12(4):958-968. doi: 10.1038/s41385-019-0172-2. Epub 2019 May 15. Mucosal Immunol. 2019. PMID: 31089187 Free PMC article.
References
- Leslie M. Cell biology: internal affairs. Science 2009;326:929–931 - PubMed
- Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 2008;8:193–204 - PubMed
- Stadnyk AW. Cytokine production by epithelial cells. FASEB J 1994;8:1041–1047 - PubMed
- Rusznak C, Sapsford RJ, Devalia JL, Shah SS, Hewitt EL, Lamont AG, Davies RJ, Lozewicz S. Interaction of cigarette smoke and house dust mite allergens on inflammatory mediator release from primary cultures of human bronchial epithelial cells. Clin Exp Allergy 2001;31:226–238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000427/TR/NCATS NIH HHS/United States
- T32 GM008692/GM/NIGMS NIH HHS/United States
- P01 HL088594/HL/NHLBI NIH HHS/United States
- 9U54TR000021/TR/NCATS NIH HHS/United States
- K23 HL081492/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous