Analysis of Fcγ receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients - PubMed (original) (raw)
Randomized Controlled Trial
. 2012 Jun 15;18(12):3478-86.
doi: 10.1158/1078-0432.CCR-11-2294. Epub 2012 Apr 13.
David J Betting, Howard M Stern, Emmanuel Quinaux, Jeremy Stinson, Somasekar Seshagiri, Ying Zhao, Marc Buyse, John Mackey, Adrian Driga, Sambasivarao Damaraju, Mark X Sliwkowski, Nicholas J Robert, Vicente Valero, John Crown, Carla Falkson, Adam Brufsky, Tadeusz Pienkowski, Wolfgang Eiermann, Miguel Martin, Valerie Bee, Omkar Marathe, Dennis J Slamon, John M Timmerman
Affiliations
- PMID: 22504044
- PMCID: PMC3821872
- DOI: 10.1158/1078-0432.CCR-11-2294
Randomized Controlled Trial
Analysis of Fcγ receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients
Sara A Hurvitz et al. Clin Cancer Res. 2012.
Abstract
Purpose: The mechanisms by which trastuzumab imparts clinical benefit remain incompletely understood. Antibody-dependent cellular cytotoxicity via interactions with Fcγ receptors (FcγR) on leukocytes may contribute to its antitumor effects. Single-nucleotide polymorphisms (SNP) in FCGR3A and FCGR2A genes lead to amino acid substitutions at positions 158 and 131, respectively, and affect binding of antibodies to FcγR such that 158V/V and 131H/H bind with highest affinity. This study aimed to determine whether high-affinity SNPs are associated with disease-free survival (DFS) among patients with HER2-positive nonmetastatic breast cancer.
Experimental design: Genomic DNA was isolated from 1,286 patients enrolled in a trial of adjuvant trastuzumab-based chemotherapy. Genotyping was conducted using Sanger sequencing and Sequenom mass spectrometry.
Results: Patient samples (N = 1,189) were successfully genotyped for FCGR3A and 1,218 for FCGR2A. Compared with the overall results of the BCIRG006 study, in the subset of patients genotyped in this analysis, a less robust improvement in DFS was observed for the trastuzumab arms than control arm (HR, 0.842; P = 0.1925). When stratified for prognostic features, the HR in favor of trastuzumab was consistent with that of the overall study (HR, 0.74; P = 0.036). No correlation between DFS and FCGR3A/2A genotypes was seen for trastuzumab-treated patients (158V/V vs. V/F vs. F/F, P = 0.98; 131H/H vs. H/R vs. R/R, P = 0.76; 158V/V and/or 131H/H vs. others, P = 0.67).
Conclusion: This analysis evaluating the association between FCGR3A/2A genotypes and trastuzumab efficacy in HER2-positive breast cancer did not show a correlation between FCGR3A-V/F and FCGR2A-H/R SNPs and DFS in patients treated with trastuzumab.
©2012 AACR.
Figures
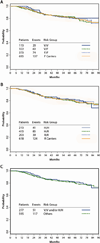
Figure 1
Consort Diagram for Adjuvant Cohort
Figure 2
Kaplan Meier estimates of disease free survival (DFS) in trastuzumab treated patients within the adjuvant cohort according to FcγR polymorphisms. (A) FCGR3A 158 V/F genotype, _P_=0.98 (V/V vs V/F vs F/F) (B) FCGR2A 131 H/R genotype, _P_=0.76 (H/H vs H/R vs R/R). (C) FcγRIIIa and FcγRIIa polymorphisms. Others represents patients with neither FCGR3A 158 V/V nor FCGR2A 131 H/H genotype, _P_=0.67 (H/H and/or V/V vs others).
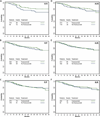
Figure 3
Kaplan-Meier estimates of disease free survival in trastuzumab (AC-TH/TCH combined) versus no trastuzumab (AC-T) treated patients, by FcγR genotype. (A) V/V (_P_=0.13) (B) V/F (_P_=0.422) (C) F/F (_P_=0.81) (D) H/H (_P_=0.35) (E) H/R (P=0.985) (F) R/R (_P_=0.58)
Similar articles
- Association studies of Fcγ receptor polymorphisms with outcome in HER2+ breast cancer patients treated with trastuzumab in NCCTG (Alliance) Trial N9831.
Norton N, Olson RM, Pegram M, Tenner K, Ballman KV, Clynes R, Knutson KL, Perez EA. Norton N, et al. Cancer Immunol Res. 2014 Oct;2(10):962-9. doi: 10.1158/2326-6066.CIR-14-0059. Epub 2014 Jul 2. Cancer Immunol Res. 2014. PMID: 24989892 Free PMC article. Clinical Trial. - Association of Polymorphisms in FCGR2A and FCGR3A With Degree of Trastuzumab Benefit in the Adjuvant Treatment of ERBB2/HER2-Positive Breast Cancer: Analysis of the NSABP B-31 Trial.
Gavin PG, Song N, Kim SR, Lipchik C, Johnson NL, Bandos H, Finnigan M, Rastogi P, Fehrenbacher L, Mamounas EP, Swain SM, Wickerham DL, Geyer CE Jr, Jeong JH, Costantino JP, Wolmark N, Paik S, Pogue-Geile KL. Gavin PG, et al. JAMA Oncol. 2017 Mar 1;3(3):335-341. doi: 10.1001/jamaoncol.2016.4884. JAMA Oncol. 2017. PMID: 27812689 Free PMC article. Clinical Trial. - ABCB1, FCGR2A, and FCGR3A polymorphisms in patients with HER2-positive metastatic breast cancer who were treated with first-line taxane plus trastuzumab chemotherapy.
Kim JW, Kim JH, Im SA, Kim YJ, Han HS, Kim JS, Han SW, Jeon YK, Oh DY, Han W, Kim TY, Park IA, Noh DY, Bang YJ. Kim JW, et al. Oncology. 2012;83(4):218-27. doi: 10.1159/000341359. Epub 2012 Aug 16. Oncology. 2012. PMID: 22906996 - Contribution of Fcgamma receptor IIIA gene 158V/F polymorphism and copy number variation to the risk of ACPA-positive rheumatoid arthritis.
Thabet MM, Huizinga TW, Marques RB, Stoeken-Rijsbergen G, Bakker AM, Kurreeman FA, White SJ, Toes RE, van der Helm-van Mil AH. Thabet MM, et al. Ann Rheum Dis. 2009 Nov;68(11):1775-80. doi: 10.1136/ard.2008.099309. Epub 2008 Nov 19. Ann Rheum Dis. 2009. PMID: 19019892 Review. - Association between functional FCGR3A F158V and FCGR2A R131H polymorphisms and responsiveness to rituximab in patients with autoimmune diseases: a meta-analysis.
Lee YH, Song GG. Lee YH, et al. Pharmacogenomics J. 2023 Nov;23(6):210-216. doi: 10.1038/s41397-023-00308-9. Epub 2023 May 6. Pharmacogenomics J. 2023. PMID: 37149714 Review.
Cited by
- FCGR3A V158F gene polymorphism and trastuzumab response in HER2-positive breast cancer patients.
Abdel-Wahed MA, Sabbour GS, Hamed AI, El Kady MS, Mohammed SK, Shaaban MAAM. Abdel-Wahed MA, et al. Sci Rep. 2024 Oct 29;14(1):26037. doi: 10.1038/s41598-024-76024-6. Sci Rep. 2024. PMID: 39472454 Free PMC article. - Serum immunoglobulin and the threshold of Fc receptor-mediated immune activation.
Bauer-Smith H, Sudol ASL, Beers SA, Crispin M. Bauer-Smith H, et al. Biochim Biophys Acta Gen Subj. 2023 Nov;1867(11):130448. doi: 10.1016/j.bbagen.2023.130448. Epub 2023 Aug 29. Biochim Biophys Acta Gen Subj. 2023. PMID: 37652365 Free PMC article. Review. - Update Breast Cancer 2022 Part 5 - Early Stage Breast Cancer.
Fehm TN, Welslau M, Müller V, Lüftner D, Schütz F, Fasching PA, Janni W, Thomssen C, Witzel I, Beierlein M, Belleville E, Untch M, Thill M, Tesch H, Ditsch N, Lux MP, Aktas B, Banys-Paluchowski M, Kolberg-Liedtke C, Hartkopf AD, Wöckel A, Kolberg HC, Harbeck N, Stickeler E. Fehm TN, et al. Geburtshilfe Frauenheilkd. 2023 Mar 9;83(3):289-298. doi: 10.1055/a-2018-9053. eCollection 2023 Mar. Geburtshilfe Frauenheilkd. 2023. PMID: 36908285 Free PMC article. - Association between genome-wide epigenetic and genetic alterations in breast cancer tissue and response to HER2-targeted therapies in HER2-positive breast cancer patients: new findings and a systematic review.
Furrer D, Dragic D, Chang SL, Fournier F, Droit A, Jacob S, Diorio C. Furrer D, et al. Cancer Drug Resist. 2022 Nov 2;5(4):995-1015. doi: 10.20517/cdr.2022.63. eCollection 2022. Cancer Drug Resist. 2022. PMID: 36627894 Free PMC article. Review. - Margetuximab Versus Trastuzumab in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results From a Randomized Phase 3 Trial.
Rugo HS, Im SA, Cardoso F, Cortes J, Curigliano G, Musolino A, Pegram MD, Bachelot T, Wright GS, Saura C, Escrivá-de-Romaní S, De Laurentiis M, Schwartz GN, Pluard TJ, Ricci F, Gwin WR 3rd, Levy C, Brown-Glaberman U, Ferrero JM, de Boer M, Kim SB, Petráková K, Yardley DA, Freedman O, Jakobsen EH, Gal-Yam EN, Yerushalmi R, Fasching PA, Kaufman PA, Ashley EJ, Perez-Olle R, Hong S, Rosales MK, Gradishar WJ; SOPHIA Study Group. Rugo HS, et al. J Clin Oncol. 2023 Jan 10;41(2):198-205. doi: 10.1200/JCO.21.02937. Epub 2022 Nov 4. J Clin Oncol. 2023. PMID: 36332179 Free PMC article. Clinical Trial.
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. - PubMed
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. - PubMed
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. - PubMed
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. - PubMed
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous