AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages - PubMed (original) (raw)
AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages
R Pierini et al. Cell Death Differ. 2012 Oct.
Abstract
The inflammasome is a signalling platform leading to caspase-1 activation. Caspase-1 causes pyroptosis, a necrotic-like cell death. AIM2 is an inflammasome sensor for cytosolic DNA. The adaptor molecule ASC mediates AIM2-dependent caspase-1 activation. To date, no function besides caspase-1 activation has been ascribed to the AIM2/ASC complex. Here, by comparing the effect of gene inactivation at different levels of the inflammasome pathway, we uncovered a novel cell death pathway activated in an AIM2/ASC-dependent manner. Francisella tularensis, the agent of tularaemia, triggers AIM2/ASC-dependent caspase-3-mediated apoptosis in caspase-1-deficient macrophages. We further show that AIM2 engagement leads to ASC-dependent, caspase-1-independent activation of caspase-8 and caspase-9 and that caspase-1-independent death is reverted upon caspase-8 inhibition. Caspase-8 interacts with ASC and active caspase-8 specifically colocalizes with the AIM2/ASC speck thus identifying the AIM2/ASC complex as a novel caspase-8 activation platform. Furthermore, we demonstrate that caspase-1-independent apoptosis requires the activation of caspase-9 and of the intrinsic pathway in a typical type II cell manner. Finally, we identify the AIM2/ASC-dependent caspase-1-independent pathway as an innate immune mechanism able to restrict bacterial replication in vitro and control IFN-γ levels in vivo in Casp1(KO) mice. This work underscores the crosstalk between inflammasome components and the apoptotic machinery and highlights the versatility of the pathway, which can switch from pyroptosis to apoptosis.
Figures
Figure 1
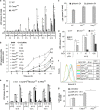
AIM2 triggers ASC-dependent, caspase-1-independent cell death upon F. novicida infection or DNA delivery into the cytosol. BMM death was assessed following U112 or ΔFPI mutant infection at the indicated MOI and time PI (a, b, e and f), following exposure to 5 _μ_M gliotoxin (c), or following cell transfection with p(dA/dT) (g). (a, c, d, e and g) Cell death was determined by quantifying LDH levels in the culture medium. Results are expressed as percentage of total LDH release. Data are shown as mean±S.E.M. (_n_=5). *P<0.05; **P<0.01. (b) Macrophages were incubated with propidium iodide (5 _μ_g/ml). Cell death was determined by measuring propidium iodide fluorescence at the indicated time of PI, using a 96-well microplate fluorimeter. (d) BMDC death following infection with U112 or ΔFPI mutant for 12 h was assessed. (f) Cell death was determined by FITC-Annexin V/propidium iodide staining in BMM infected by U112 (MOI 100, 8 h PI). FITC fluorescence levels in propidium iodide-negative cells are shown, mean fluorescence intensity (MFI) is indicated. One experiment representative of three independent experiments is shown
Figure 2
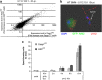
Differential cell death between Casp1KO and ASCKO BMM is not associated with differences in transcriptional responses or AIM2 activation. (a) Transcriptional changes as determined by microarray analysis in U112-infected Casp1KO (plotted on the x axis) and ASCKO BMM (plotted on the y axis) are shown. Each point represents expression level of single transcript at 6 h PI (MOI 100) normalised to its expression in the corresponding uninfected BMM. (b) Example of GFP-AIM2 speck formation in BMM following U112 infection. GFP-AIM2-expressing BMM (ASCKO in the figure) were infected with U112 for 6 h (MOI 10). U112 bacteria were immunostained with a polyclonal anti-F. novicida antibody (red), and nuclei were labelled with DAPI. On the left, an infected BMM displayed diffuse cytosolic GFP-AIM2 pattern (no activation) while the cell on the right showed AIM2 activation as visualised by GFP-AIM2 relocalisation into a speck. (c) Speck-forming Casp1KO and ASCKO BMM following ΔFPI mutant or U112 infection were counted under the microscope. Results are expressed as mean±S.E.M. (_n_=3) and are representative of three independent experiments
Figure 3
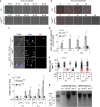
_F. novicida_-infected Casp1KO BMM die in an apoptotic manner. (a) Analysis of changes in cell morphology and cell membrane permeabilisation were investigated by time-lapse video microscopy in WT and Casp1KO BMM, infected with U112 (MOI 10). Loss of integrity of the plasma membrane was visualised by EtDi fluorescence. Infection by GFP-expressing U112 was checked at the first time point thanks to GFP signal. (b) Detection of inflammasome speck formation and observation of changes in cell morphology in WT and Casp1KO BMM following transfection with p(dA/dT) were performed by time-lapse investigation of ASC-mCherry-transduced macrophages. Apparent large size of ASC-mCherry speck is owing to the overexposure required to visualise the weaker ASC-mCherry diffuse signal. (c) Delay between cell shrinkage, nuclear condensation and cell membrane permeabilisation was observed in Casp1KO BMM, but not in WT and ASCKO BMM. Condensed nuclei had a reduced size and non-detectable nucleoles by DAPI staining. Cell permeabilisation was observed by EtDi staining. (d) Cells with condensed nuclei and intact cell membrane (no EtDi staining) were quantified by microscopy. Results are expressed as mean±S.E.M. (_n_=3), and are representative of three independent experiments. *P<0.05; **P<0.01. (e) Nucleus size was determined at 10 h PI using Image J software. Nuclei were classified as either EtDi positive (EtDi+) or negative (EtDi−). EtDi−, condensed nuclei, which are highlighted in the red squares were more prevalent in Casp1KO than in WT BMM. (f) TUNEL assay was performed on macrophages infected with the indicated strains at the indicated MOI and at the indicated time of PI. The percentage of cells containing TUNEL+ nuclei was quantified by microscopy. Results are expressed as mean±S.E.M. (_n_=3), and are representative of three independent experiments. *P<0.05; **P<0.01. (g) Ladder-like DNA fragmentation was observed in Casp1KO BMM, but not in WT and ASCKO, infected with U112 (MOI 100) for 8 h. Conversely, DNA laddering was observed in WT, Casp1KO and ASCKO macrophages after a 5-h treatment with 5 _μ_M gliotoxin. Fragmented soluble DNA from 106 BMM was loaded in each lane. Results are from one experiment representative of three independent experiments. NT, not tested
Figure 4
Apoptosis in Casp1KO BMM following F. novicida infection is mediated by caspase-3. (a) Caspase-3/7 activity was investigated by measuring fluorescence signal emitted upon Ac-DEVD-AMC hydrolysis when incubated with protein lysates from U112-infected WT (6 h PI), Casp1KO and ASCKO BMM (8 h PI). (b) Caspase-3 processing was monitored by western blot analysis in WT BMM infected with U112 or ΔFPI mutant for 5 h, and Casp1KO and ASCKO BMM infected for 6 h. (c) Effect of caspase-3 inhibitor Z-DEVD-FMK in reverting Casp1KO BMM death following infection with U112 for 10 h. Results are expressed as percentage of total LDH release. Data are shown as mean±S.E.M. (_n_=3), *P<0.05. Results are representative of three independent experiments
Figure 5
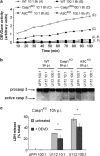
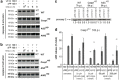
Caspase-8 and -9 are processed in _F. novicida_-infected Casp1KO macrophages and their inhibition reverts caspase-1-independent cell death. Caspase-8 (a) and -9 (b) cleavage were investigated by immunoblot analysis in WT, Casp1KO and ASCKO BMM infected with U112 (MOI 100 : 1) at the indicated time post-infection or ΔFPI mutant (MOI 100 : 1) at 8 h PI. The 8 h PI time point was not investigated in WT macrophages due to high cell death level. ‘8#': ΔFPI mutant (MOI 100 : 1) -infected WT BMM were analysed at 6 h PI. (c) Decrease in procaspase-2 levels was assessed in U112- or ΔFPI mutant-infected WT BMM at 7 h PI, and Casp1KO and ASCKO BMM at 8 h PI. Procaspase-2 quantification normalised to ΔFPI-infected control is shown. (d) Cell death levels were determined by LDH assay in _F. novicida_-infected Casp1KO BMM upon treatment with caspase-2 (Z-VDVAD-FMK), caspase-8 (Z-IETD-FMK) and caspase-9 (Ac-LEHD-CMK) inhibitors. Data are shown as mean±S.E.M. (_n_=3). *P<0.01. One experiment representative of three independent experiments is shown. NT, not tested
Figure 6
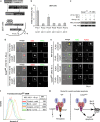
Caspase-8 functionally interacts with ASC within the AIM2/ASC speck. (a) Diagram of inflammasome-like pathway reconstitution in 293T cells. (b) The different initiator caspase prodomains (CARD domains for caspase-1, -2, -9, -11, -12 and DED domain for caspase-8) were fused to the active domain of caspase-1 and screened for functional interaction with the AIM2/ASC complex. Inflammasome-like pathway was reconstituted by cotransfecting 293T cells with plasmids expressing AIM2, ASC, pro-IL-1_β_ and either of the chimeric caspases. Interaction of ASC with CARDcasp1 and DEDcasp8 chimeric proteins led to IL-1_β_ release. Analysis of IL-1_β_ concentration in culture medium of 293T was determined by ELISA. Results are presented as IL-1_β_ concentration fold increase between 293T cotransfected with AIM2, ASC, pro-IL-1_β_ and either of the chimeric proteins versus cells undergoing the same cotransfection except for the absence of ASC-encoding plasmid. Raw values were typically 50 pg/ml of IL-1_β_ in the absence of ASC and reached 250 pg/ml upon ASC addition. Data are shown as mean±S.E.M. (_n_=9). *P<0.05. One experiment representative of three independent experiments is shown. (c) Casp1KO macrophages were infected with U112 (MOI 100 : 1) for the indicated times. When indicated, the caspase-8 inhibitor (z-IETD-FMK at 50 _μ_M) was added at 1 h PI. Endogenous ASC protein was immunoprecipitated from 107 cells. The level of ASC and caspase-8 (co)immunoprecipitated were analysed by immunoblot. One experiment out of two independent experiments is shown. (d) Casp1KO macrophages were infected for 8 h with U112 at a MOI of 10 : 1. Endogenous ASC (top panel) and AIM2 (lower panel) proteins were detected by immunofluorescence. Active caspase-8 and caspase-9 were detected by a FITC-IETD and a FITC-LEHD probes, respectively. (d and e) Image dimensions are as follows: 30 × 30 _μ_m2. The insets (3 × 3 _μ_m2) correspond to 10-fold magnification of the ASC or Aim2 specks. (e) WT (top panel) and Casp1KO (bottom panel) macrophages were transduced with lentiviruses encoding FLAG-Casp8-DED domain or FLAG-Casp9-CARD domain. 96 h post-transduction, macrophages were infected with U112 at a MOI of 10 : 1 for 8 h. Endogenous ASC protein and ectopically expressed FLAG-tagged-fusion proteins were detected by immunofluorescence. (f) Casp1KO macrophages were transduced with Bcl-2-encoding or control lentiviruses. 96 h post-transduction, macrophages were infected with U112 at a MOI of 100 : 1 for 6 h. Cell death was determined by flow cytometry following annexin V-APC staining. Doublets and cell debris were excluded from the analysis based on FSC-H, FSC-A and SSC-A parameters. (g) Model for AIM2/ASC cell death pathways in WT and Casp1KO macrophages (see text for details)
Figure 7
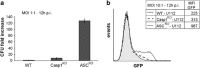
The ASC-dependent apoptosis restricts intracellular F. novicida replication in Casp1KO macrophages in vitro. (a) Analysis of intracellular bacterial population in WT, Casp1KO and ASCKO BMM, after cell infection with U112 (MOI 1) for 12 h. Results are shown as mean±S.E.M. (_n_=3) and are representative of three independent experiments. (b) Bacterial replication was assessed by measuring GFP-fluorescence levels emitted by intracellular GFP-expressing U112 (MOI 10) at 12 h PI in WT, Casp1KO and ASCKO BMM
Figure 8
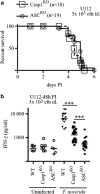
An ASC-dependent pathway controls IFN-γ levels during tularaemia in Casp1KO mice. (a) Casp1KO mice (_n_=18, dashed line, square symbols) and ASCKO mice (_n_=19, plain line, round symbols) were infected with 5 × 103 F. novicida strain U112 CFU intradermally. Survival was monitored twice a day for 6 days. (b) WT, Casp1KO and ASCKO mice were infected with 5 × 103 U112 CFU. IFN-γ levels in serum were determined at 48 h PI by multiplex immunoassay. Each point represents the value obtained for one mouse. Geometric mean is shown. Mann–Whitney statistical analysis was performed. Two-tailed _P_-value is shown
Similar articles
- ASC controls IFN-γ levels in an IL-18-dependent manner in caspase-1-deficient mice infected with Francisella novicida.
Pierini R, Perret M, Djebali S, Juruj C, Michallet MC, Förster I, Marvel J, Walzer T, Henry T. Pierini R, et al. J Immunol. 2013 Oct 1;191(7):3847-57. doi: 10.4049/jimmunol.1203326. Epub 2013 Aug 23. J Immunol. 2013. PMID: 23975862 - Caspase-1 activity affects AIM2 speck formation/stability through a negative feedback loop.
Juruj C, Lelogeais V, Pierini R, Perret M, Py BF, Jamilloux Y, Broz P, Ader F, Faure M, Henry T. Juruj C, et al. Front Cell Infect Microbiol. 2013 Apr 24;3:14. doi: 10.3389/fcimb.2013.00014. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23630667 Free PMC article. - The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses.
Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. Rathinam VA, et al. Nat Immunol. 2010 May;11(5):395-402. doi: 10.1038/ni.1864. Epub 2010 Mar 28. Nat Immunol. 2010. PMID: 20351692 Free PMC article. - Francisella tularensis: activation of the inflammasome.
Weiss DS, Henry T, Monack DM. Weiss DS, et al. Ann N Y Acad Sci. 2007 Jun;1105:219-37. doi: 10.1196/annals.1409.005. Epub 2007 Mar 29. Ann N Y Acad Sci. 2007. PMID: 17395724 Review. - Regulating caspase-1 during infection: roles of NLRs, AIM2, and ASC.
Case CL. Case CL. Yale J Biol Med. 2011 Dec;84(4):333-43. Yale J Biol Med. 2011. PMID: 22180671 Free PMC article. Review.
Cited by
- Implications of inflammatory cell death-PANoptosis in health and disease.
Bae H, Jang Y, Karki R, Han JH. Bae H, et al. Arch Pharm Res. 2024 Jul;47(7):617-631. doi: 10.1007/s12272-024-01506-0. Epub 2024 Jul 10. Arch Pharm Res. 2024. PMID: 38987410 Review. - The nonreceptor tyrosine kinase SYK drives caspase-8/NLRP3 inflammasome-mediated autoinflammatory osteomyelitis.
Dasari TK, Geiger R, Karki R, Banoth B, Sharma BR, Gurung P, Burton A, Kanneganti TD. Dasari TK, et al. J Biol Chem. 2020 Mar 13;295(11):3394-3400. doi: 10.1074/jbc.RA119.010623. Epub 2019 Nov 12. J Biol Chem. 2020. PMID: 31719149 Free PMC article. - Functional Assessment of Disease-Associated Pyrin Variants.
Chirita D, Jamilloux Y, Henry T, Magnotti F. Chirita D, et al. Methods Mol Biol. 2022;2523:179-195. doi: 10.1007/978-1-0716-2449-4_12. Methods Mol Biol. 2022. PMID: 35759198 - A long-awaited merger of the pathways mediating host defence and programmed cell death.
Blander JM. Blander JM. Nat Rev Immunol. 2014 Sep;14(9):601-18. doi: 10.1038/nri3720. Nat Rev Immunol. 2014. PMID: 25145756 Review. - Identification of multifaceted binding modes for pyrin and ASC pyrin domains gives insights into pyrin inflammasome assembly.
Vajjhala PR, Kaiser S, Smith SJ, Ong QR, Soh SL, Stacey KJ, Hill JM. Vajjhala PR, et al. J Biol Chem. 2014 Aug 22;289(34):23504-19. doi: 10.1074/jbc.M114.553305. Epub 2014 Jul 8. J Biol Chem. 2014. PMID: 25006247 Free PMC article.
References
- Bielecki J, Youngman P, Connelly P, Portnoy DA. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. - PubMed
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. - PubMed
- Brodsky IE, Monack D. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Semin Immunol. 2009;21:199–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous