Elevated PGC-1α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS - PubMed (original) (raw)
. 2012 May 2;15(5):778-86.
doi: 10.1016/j.cmet.2012.03.019.
Philippe A Parone, Vanda S Lopes, Concepción Lillo, Melissa McAlonis-Downes, Sandra K Lee, Anne P Vetto, Susanna Petrosyan, Martin Marsala, Anne N Murphy, David S Williams, Bruce M Spiegelman, Don W Cleveland
Affiliations
- PMID: 22560226
- PMCID: PMC3565468
- DOI: 10.1016/j.cmet.2012.03.019
Elevated PGC-1α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS
Sandrine Da Cruz et al. Cell Metab. 2012.
Abstract
The transcriptional coactivator PGC-1α induces multiple effects on muscle, including increased mitochondrial mass and activity. Amyotrophic lateral sclerosis (ALS) is a progressive, fatal, adult-onset neurodegenerative disorder characterized by selective loss of motor neurons and skeletal muscle degeneration. An early event is thought to be denervation-induced muscle atrophy accompanied by alterations in mitochondrial activity and morphology within muscle. We now report that elevation of PGC-1α levels in muscles of mice that develop fatal paralysis from an ALS-causing SOD1 mutant elevates PGC-1α-dependent pathways throughout disease course. Mitochondrial biogenesis and activity are maintained through end-stage disease, accompanied by retention of muscle function, delayed muscle atrophy, and significantly improved muscle endurance even at late disease stages. However, survival was not extended. Therefore, muscle is not a primary target of mutant SOD1-mediated toxicity, but drugs increasing PGC-1α activity in muscle represent an attractive therapy for maintaining muscle function during progression of ALS.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
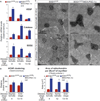
Figure 1. Elevating PGC-1α Expression Activates Known PGC-1α Responsive Pathways, Including Mitochondrial Biogenesis in Skeletal Muscles of SOD1G37R Mutant Mice throughout Disease
(A) Relative mRNA expression levels were determined by quantitative real-time PCR for VEGF, catalase, and SOD2 in gastrocnemius isolated from SOD1G37R and SOD1G37R/MCK-PGC-1α animals throughout disease. Data are presented as mean ± SEM. See also Figure S1. (B) Relative numbers of acetylcholine receptor (AChR) clusters per section of gastrocnemius were determined by staining with α-bungarotoxin. Data are presented as mean ± SEM. (C) Electron micrographs from cross-sections taken from tibialis anterior muscle of SOD1G37R and SOD1G37R/MCK-PGC-1α animals at asymptomatic (4 months) and end-stage of disease (13–14 months). Scale bar = 0.5 µm. (D) Total area of mitochondria per 20 µm2 of myofiber from tibialis anterior muscle of SOD1G37R and SOD1G37R/MCK-PGC-1α animals at asymptomatic (4 months), symptomatic (12 months), and end-stage of disease (13–14 months). Data are presented as mean ± SEM.
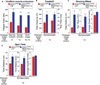
Figure 2. Elevating PGC-1α Expression Improves Muscle Activity and Locomotive Activity in Mutant SOD1G37R Mice
(A) Fatigue index of hindlimb muscles from SOD1G37R and SOD1G37R/MCK-PGC-1α animals throughout disease. Fatigue index was quantified as the period (in seconds) of high-frequency electrical stimulation required to obtain a 50% decrease in muscle contraction. Data are presented as mean ± SEM. See also Figure S2. (B) Treadmill performance (total distance and run time on the treadmill determined until exhaustion) of SOD1G37R and SOD1G37R/MCK-PGC-1α animals at the symptomatic stage of disease. Data are presented as mean ± SEM. (C) Running wheel performance of SOD1G37R and SOD1G37R/MCK-PGC-1α animals at the symptomatic stage of disease. Total distance and run time on the wheel were determined during a 5 min testing period. Average speed corresponds to the mean speed of running during the mobile period. Data are presented as mean ± SEM. (D) Open field performance of SOD1G37R and SOD1G37R/MCK-PGC-1α animals at the symptomatic stage of disease. Total distance covered and the time mobile were determined during a 60 min of tracking period. Average speed corresponds to the mean speed of movement during the mobile period. Data are presented as mean ± SEM.
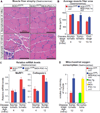
Figure 3. Elevating PGC-1α Expression Reduces Muscle Atrophy and Expression of Muscle Degeneration Genes and Increases Overall Muscle Mitochondrial ADP Phosphorylation Capacity in Mutant SOD1G37R Mice throughout Disease
(A) Representative hematoxylin and eosin stainings of the gastrocnemius muscle in asymptomatic and symptomatic SOD1G37R and SOD1G37R/MCK-PGC-1α animals. The inset indicates clusters of small angular degenerating fibers. (B) Quantification of average fiber area from hematoxylin and eosin-stained gastrocnemius muscle from SOD1G37R and SOD1G37R/MCK-PGC-1α animals throughout disease. Data are presented as mean ± SEM. See also Figure S3. (C) Relative mRNA expression levels of muscular degeneration markers MuRF-1 and cathepsin L in gastrocnemius muscle isolated from asymptomatic and symptomatic mutant SOD1G37R and SOD1G37R/MCK-PGC-1α animals. mRNA expression was evaluated by quantitative real-time PCR. Data are presented as mean ± SEM. (D) Levels of ADP-stimulated mitochondrial oxygen consumption in mitochondria isolated from the gastrocnemius muscle of symptomatic mutant SOD1G37R and SOD1G37R/MCK-PGC-1α and age-matched nontransgenic and MCK-PGC-1α animals oxidizing pyruvate and malate. In coupled mitochondrial respiration, oxygen consumption is directly proportional to the amount of ATP synthesized. Data are presented as mean ± SEM.
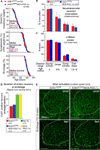
Figure 4. Elevating PGC-1α Expression in Skeletal Muscle of SOD1G37R Mutant Mice Does Not Alter ALS Disease Course or Pathogenesis
(A) Plot of ages (in days) at which disease onset (as determined by the weight peak; at onset, animals do not display any obvious motor phenotype), symptomatic stage (as determined by 10% weight loss from onset, a stage characterized by clear gait abnormalities and tremor) and end-stage (as determined by hindlimb paralysis and inability to right itself) were reached for SOD1G37R (red) and SOD1G37R/MCK-PGC-1α (blue) animals. (B–D) Quantification of innervation at the neuromuscular junction of the gastrocnemius muscle (B), total number of α-motor axons in the lumbar L5 motor root (C), and quantification at disease end-stage of the average number of large cholinergic ventral horn motor neurons per section of lumbar spinal cord from SOD1G37R and SOD1G37R/MCK-PGC-1α animals (D). Data are presented as mean ± SEM. See also Figure S4 for representative sections of the spinal cords used for quantification. (E) Representative micrographs of lumbar spinal cord sections from SOD1G37R and SOD1G37R/MCK-PGC-1α animals at disease end-stage processed for immunofluorescence using antibodies detecting activated astrocytes (GFAP) or microglia (IbaI). Dashed outlines correspond to the boundary between gray and white matter.
Comment in
- Muscling in on PGC-1α for improved quality of life in ALS.
Johri A, Beal MF. Johri A, et al. Cell Metab. 2012 May 2;15(5):567-9. doi: 10.1016/j.cmet.2012.04.015. Cell Metab. 2012. PMID: 22560208
Similar articles
- Peroxisome Proliferator Activator Receptor Gamma Coactivator-1α Overexpression in Amyotrophic Lateral Sclerosis: A Tale of Two Transgenics.
Varghese M, Zhao W, Trageser KJ, Pasinetti GM. Varghese M, et al. Biomolecules. 2020 May 13;10(5):760. doi: 10.3390/biom10050760. Biomolecules. 2020. PMID: 32414179 Free PMC article. - Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis.
Russell AP, Wada S, Vergani L, Hock MB, Lamon S, Léger B, Ushida T, Cartoni R, Wadley GD, Hespel P, Kralli A, Soraru G, Angelini C, Akimoto T. Russell AP, et al. Neurobiol Dis. 2013 Jan;49:107-17. doi: 10.1016/j.nbd.2012.08.015. Epub 2012 Sep 4. Neurobiol Dis. 2013. PMID: 22975021 - Decreased mRNA expression of PGC-1α and PGC-1α-regulated factors in the SOD1G93A ALS mouse model and in human sporadic ALS.
Thau N, Knippenberg S, Körner S, Rath KJ, Dengler R, Petri S. Thau N, et al. J Neuropathol Exp Neurol. 2012 Dec;71(12):1064-74. doi: 10.1097/NEN.0b013e318275df4b. J Neuropathol Exp Neurol. 2012. PMID: 23147503 - PGC-1alpha: turbocharging mitochondria.
Houten SM, Auwerx J. Houten SM, et al. Cell. 2004 Oct 1;119(1):5-7. doi: 10.1016/j.cell.2004.09.016. Cell. 2004. PMID: 15454076 Review.
Cited by
- S[+] Apomorphine is a CNS penetrating activator of the Nrf2-ARE pathway with activity in mouse and patient fibroblast models of amyotrophic lateral sclerosis.
Mead RJ, Higginbottom A, Allen SP, Kirby J, Bennett E, Barber SC, Heath PR, Coluccia A, Patel N, Gardner I, Brancale A, Grierson AJ, Shaw PJ. Mead RJ, et al. Free Radic Biol Med. 2013 Aug;61:438-52. doi: 10.1016/j.freeradbiomed.2013.04.018. Epub 2013 Apr 19. Free Radic Biol Med. 2013. PMID: 23608463 Free PMC article. - Therapeutic neuroprotective agents for amyotrophic lateral sclerosis.
Pandya RS, Zhu H, Li W, Bowser R, Friedlander RM, Wang X. Pandya RS, et al. Cell Mol Life Sci. 2013 Dec;70(24):4729-45. doi: 10.1007/s00018-013-1415-0. Epub 2013 Jul 18. Cell Mol Life Sci. 2013. PMID: 23864030 Free PMC article. Review. - PGC-1α in the myofibers regulates the balance between myogenic and adipogenic progenitors affecting muscle regeneration.
Beltrà M, Pin F, Costamagna D, Duelen R, Renzini A, Ballarò R, Garcia-Castillo L, Iannuzzi A, Moresi V, Coletti D, Sampaolesi M, Penna F, Costelli P. Beltrà M, et al. iScience. 2022 Nov 2;25(11):105480. doi: 10.1016/j.isci.2022.105480. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388980 Free PMC article. - PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders.
Jamwal S, Blackburn JK, Elsworth JD. Jamwal S, et al. Pharmacol Ther. 2021 Mar;219:107705. doi: 10.1016/j.pharmthera.2020.107705. Epub 2020 Oct 9. Pharmacol Ther. 2021. PMID: 33039420 Free PMC article. Review. - Mitochondrial dysfunction in neurodegenerative diseases.
Johri A, Beal MF. Johri A, et al. J Pharmacol Exp Ther. 2012 Sep;342(3):619-30. doi: 10.1124/jpet.112.192138. Epub 2012 Jun 13. J Pharmacol Exp Ther. 2012. PMID: 22700435 Free PMC article. Review.
References
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. - PubMed
- Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. - PubMed
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. - PubMed
- Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, Flint Beal M, Manfredi G. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J. Neurochem. 2006;96:1349–1361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous