Global heterochromatin loss: a unifying theory of aging? - PubMed (original) (raw)
Global heterochromatin loss: a unifying theory of aging?
Amy Tsurumi et al. Epigenetics. 2012 Jul.
Abstract
The aging field is replete with theories. Over the past years, many distinct, yet overlapping mechanisms have been proposed to explain organismal aging. These include free radicals, loss of heterochromatin, genetically programmed senescence, telomere shortening, genomic instability, nutritional intake and growth signaling, to name a few. The objective of this Point-of-View is to highlight recent progress on the "loss of heterochromatin" model of aging and to propose that epigenetic changes contributing to global heterochromatin loss may underlie the various cellular processes associated with aging.
Figures
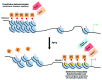
Figure 1. Heterochromatin redistribution during aging. Aging is associated with a loss of constitutive heterochromatin, as demonstrated by a decrease in H3K9 methylation and delocalization of HP1 proteins. Concurrently, increases in facultative heterochromatin occur at specific loci, particularly at Senescence-Associated Heterochromatin Foci (SAHFs) that depend on the activity of H3K9 methyltransferases, on recruitment of HP1 proteins and histone chaperones, and on the presence of macro H2A. The decrease in activity of the maintenance DNA methyltransferase Dnmt1 and the increase in de novo Dnmt3 methyltransferases during aging may also contribute to heterochromatin redistribution.
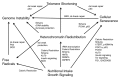
Figure 2. Epigenetic changes resulting in a decrease and redistribution of global heterochromatin may underlie the various models of aging. The models of free radical accumulation, genetically programmed senescence, telomere shortening, genomic instability, nutritional intake and growth signaling are distinct, yet overlapping theories of aging that may all be linked by heterochromatin redistribution.
Similar articles
- Genomic Instability and Epigenetic Changes during Aging.
López-Gil L, Pascual-Ahuir A, Proft M. López-Gil L, et al. Int J Mol Sci. 2023 Sep 19;24(18):14279. doi: 10.3390/ijms241814279. Int J Mol Sci. 2023. PMID: 37762580 Free PMC article. Review. - Markers of cellular senescence. Telomere shortening as a marker of cellular senescence.
Bernadotte A, Mikhelson VM, Spivak IM. Bernadotte A, et al. Aging (Albany NY). 2016 Jan;8(1):3-11. doi: 10.18632/aging.100871. Aging (Albany NY). 2016. PMID: 26805432 Free PMC article. Review. - Molecular Aspects of Senescence and Organismal Ageing-DNA Damage Response, Telomeres, Inflammation and Chromatin.
Sławińska N, Krupa R. Sławińska N, et al. Int J Mol Sci. 2021 Jan 8;22(2):590. doi: 10.3390/ijms22020590. Int J Mol Sci. 2021. PMID: 33435578 Free PMC article. Review. - Aging Triggers H3K27 Trimethylation Hoarding in the Chromatin of Nothobranchius furzeri Skeletal Muscle.
Cencioni C, Heid J, Krepelova A, Rasa SMM, Kuenne C, Guenther S, Baumgart M, Cellerino A, Neri F, Spallotta F, Gaetano C. Cencioni C, et al. Cells. 2019 Sep 28;8(10):1169. doi: 10.3390/cells8101169. Cells. 2019. PMID: 31569376 Free PMC article. - Heterochromatin assembly: a new twist on an old model.
Horn PJ, Peterson CL. Horn PJ, et al. Chromosome Res. 2006;14(1):83-94. doi: 10.1007/s10577-005-1018-1. Chromosome Res. 2006. PMID: 16506098 Review.
Cited by
- Lithium inhibits oxidative stress-induced neuronal senescence through miR-34a.
Tufekci KU, Alural B, Tarakcioglu E, San T, Genc S. Tufekci KU, et al. Mol Biol Rep. 2021 May;48(5):4171-4180. doi: 10.1007/s11033-021-06430-w. Epub 2021 May 30. Mol Biol Rep. 2021. PMID: 34052966 - Hypoxia-Inducible Histone Lysine Demethylases: Impact on the Aging Process and Age-Related Diseases.
Salminen A, Kaarniranta K, Kauppinen A. Salminen A, et al. Aging Dis. 2016 Mar 15;7(2):180-200. doi: 10.14336/AD.2015.0929. eCollection 2016 Mar. Aging Dis. 2016. PMID: 27114850 Free PMC article. Review. - Sex gap in aging and longevity: can sex chromosomes play a role?
Marais GAB, Gaillard JM, Vieira C, Plotton I, Sanlaville D, Gueyffier F, Lemaitre JF. Marais GAB, et al. Biol Sex Differ. 2018 Jul 17;9(1):33. doi: 10.1186/s13293-018-0181-y. Biol Sex Differ. 2018. PMID: 30016998 Free PMC article. Review. - Poly(A) RNA sequencing reveals age-related differences in the prefrontal cortex of dogs.
Sándor S, Jónás D, Tátrai K, Czeibert K, Kubinyi E. Sándor S, et al. Geroscience. 2022 Jun;44(3):1269-1293. doi: 10.1007/s11357-022-00533-3. Epub 2022 Mar 14. Geroscience. 2022. PMID: 35288843 Free PMC article. - Genomic Instability and Epigenetic Changes during Aging.
López-Gil L, Pascual-Ahuir A, Proft M. López-Gil L, et al. Int J Mol Sci. 2023 Sep 19;24(18):14279. doi: 10.3390/ijms241814279. Int J Mol Sci. 2023. PMID: 37762580 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous