Mll partial tandem duplication and Flt3 internal tandem duplication in a double knock-in mouse recapitulates features of counterpart human acute myeloid leukemias - PubMed (original) (raw)
. 2012 Aug 2;120(5):1130-6.
doi: 10.1182/blood-2012-03-415067. Epub 2012 Jun 6.
Kelsie M Bernot, Susan P Whitman, Ronald F Siebenaler, Elshafa H Ahmed, Gabriele G Marcucci, Daniel A Yanes, Kathleen K McConnell, Charlene Mao, Chidimma Kalu, Xiaoli Zhang, David Jarjoura, Adrienne M Dorrance, Nyla A Heerema, Benjamin H Lee, Gang Huang, Guido Marcucci, Michael A Caligiuri
Affiliations
- PMID: 22674806
- PMCID: PMC3412333
- DOI: 10.1182/blood-2012-03-415067
Mll partial tandem duplication and Flt3 internal tandem duplication in a double knock-in mouse recapitulates features of counterpart human acute myeloid leukemias
Nicholas A Zorko et al. Blood. 2012.
Abstract
The MLL-partial tandem duplication (PTD) associates with high-risk cytogenetically normal acute myeloid leukemia (AML). Concurrent presence of FLT3-internal tandem duplication (ITD) is observed in 25% of patients with MLL-PTD AML. However, mice expressing either Mll-PTD or Flt3-ITD do not develop AML, suggesting that 2 mutations are necessary for the AML phenotype. Thus, we generated a mouse expressing both Mll-PTD and Flt3-ITD. Mll(PTD/WT):Flt3(ITD/WT) mice developed acute leukemia with 100% penetrance, at a median of 49 weeks. As in human MLL-PTD and/or the FLT3-ITD AML, mouse blasts exhibited normal cytogenetics, decreased Mll-WT-to-Mll-PTD ratio, loss of the Flt3-WT allele, and increased total Flt3. Highlighting the adverse impact of FLT3-ITD dosage on patient survival, mice with homozygous Flt3-ITD alleles, Mll(PTD/WT):Flt3(ITD/ITD), demonstrated a nearly 30-week reduction in latency to overt AML. Here we demonstrate, for the first time, that Mll-PTD contributes to leukemogenesis as a gain-of-function mutation and describe a novel murine model closely recapitulating human AML.
Figures
Figure 1
MllPTD/WT:_Flt3_ITD/WT mice develop a predominance of AML. (A) Frequencies of acute leukemia immunophenotypes identified in _Mll_PTD/WT:_Flt3_ITD/WT mice (n = 33). (B) Representative flow cytometry plots from spleen and BM samples from age-matched controls and moribund _Mll_PTD/WT:_Flt3_ITD/WT mice killed with elevated WBC counts. AML with maturation (myeloperoxidase [MPO+]/CD3−/CD19−/B220−/CD11b+/Gr1+/CD117−; 15%), AML without maturation group A (MPO+/CD3−/B220lo/CD19−/CD11blo/−/Gr1−/CD117+/−; 34%), AML without maturation group B (MPO+/CD3−/B220+/CD19−/CD11b+/−/Gr1−/CD117+/−; 21%). Regions highlighted in the red rectangle emphasize key differences in populations for each of the AML immunophenotypes. MPO staining by IHC and flow cytometry results for B-cell and biphenotypic leukemias are not shown. (C) WBC counts of leukemic _Mll_PTD/WT:_Flt3_ITD/WT mice are significantly elevated at the time of death. Figure shows WBC counts from 3 age-matched cohorts of _Mll_PTD/WT:_Flt3_ITD/WT mice with AML and controls. (D) _Mll_PTD/WT:_Flt3_ITD/WT mice with leukemia exhibit significant splenomegaly at the time of death. Figure shows weights from 3 age-matched cohorts of _Mll_PTD/WT:_Flt3_ITD/WT mice with AML and controls. (E) Representative image of spleens from a _Mll_PTD/WT:_Flt3_ITD/WT mouse dying of AML with age-matched controls. (F) Representative blood smear stained with Wright-Giemsa and hematoxylin and eosin-stained organ preparations from a _Mll_PTD/WT:_Flt3_ITD/WT mouse dying of AML. Micrographs demonstrate blasts in peripheral blood (original magnification ×1000) and extensive infiltrations of myeloid blasts in liver (original magnification ×400), and adrenal gland (original magnification ×400). Micrograph images were recorded using a Zeiss Axioskop, Olympus Magnafire digital camera, using a 10× eyepiece, 40×/0.65 NA Acroplan objective lens or 100×/1.40 NA Plan-Apochromat and Zeiss Axiovision Version 4.7.2 software.
Figure 2
BM samples from _Mll_PTD/WT:_Flt3_ITD/WT mice dying of AML are cytogenetically normal as are the age-matched, WT, and single-mutant controls. G-banded karyotype analysis of WT, single-mutant, and leukemic _Mll_PTD/WT:_Flt3_ITD/WT mice was conducted on whole BM samples.
Figure 3
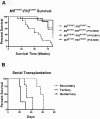
MllPTD/WT:_Flt3_ITD/WT mice develop a fatal AML that is transplantable. (A) Survival of WT and single knock-in (MllPTD/WT:_Flt3_WT/WT or _Mll_WT/WT:_Flt3_ITD/WT) control mice and mice with the heterozygous alleles of _Mll_PTD/WT:_Flt3_ITD/WT. (B) _Mll_PTD/WT:_Flt3_ITD/WT cells from spleen containing AML are serially transplantable and lethal. Serially transplanted cells develop into progressively more aggressive AML with each successive passage.
Figure 4
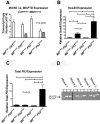
AML from _Mll_PTD/WT:_Flt3_ITD/WT mice have molecular features similar to those reported for human MLL-PTD+ and/or FLT3-ITD+ AML. (A) Quantitative real-time RT-PCR was performed using CD117+ BM cells sorted to > 95% purity from age-matched mice ≥ 50 weeks of age to measure the copy numbers of the mouse _Mll_-PTD and the _Mll-_WT transcripts. Results demonstrate reduced _Mll_-WT copy number only in leukemic _Mll_PTD/WT:_Flt3_ITD/WT mice. Figure is representative of 2 age-matched cohorts of mice. (B) Relative real-time RT-PCR measuring HoxA9 expression in whole BM cells from age-matched mice > 50 weeks of age. (C) Total Flt3 mRNA was measured by relative real-time RT-PCR using BM cells of age-matched mice ≥ 50 weeks of age. (D) Genomic PCR reactions using 150 ng DNA and genotyping primers to amplify both the _Flt3_-ITD and _Flt3_-WT loci were performed to demonstrate a reduction or loss of _Flt3_-WT in primary _Mll_PTD/WT:_Flt3_ITD/WT AML samples taken from moribund mice. Multiple samples from each animal are defined as follows: lane 1, tail DNA from genotyping at 4 weeks; lane 2, whole BM DNA from the time of death when moribund with AML (50-80 weeks); and lane 3, sorted leukemic Ly5.2 _Mll_PTD/WT:_Flt3_ITD/WT AML blasts from secondary transplant of _Mll_PTD/WT:_Flt3_ITD/WT AML blasts into Ly5.1 recipients. The Ly5.2 → Ly5.1 adoptive transfer was used to obtain a pure population of _Mll_PTD/WT:_Flt3_ITD/WT AML blasts for analysis. Five representative samples are shown. Reactions that did not produce any bands for _Flt3_-WT and _Flt3_-ITD were removed as noted by the black bar.
Figure 5
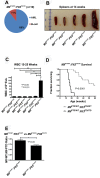
Acute leukemia in _Mll_PTD/WT:_Flt3_ITD/ITD mice. (A) Only AML and B-cell leukemias developed in _Mll_PTD/WT:_Flt3_ITD/ITD mice. Nineteen mice were analyzed. (B) _Mll_PTD/WT:_Flt3_ITD/ITD mice with leukemia exhibited significant splenomegaly at time of death. (C) WBC counts (median ± SEM) for leukemic _Mll_PTD/WT:_Flt3_ITD/ITD and age-matched control genotypes at the time of death. (D) _Mll_PTD/WT:_Flt3_ITD/ITD mice died of AML with a significantly shorter latency than _Mll_PTD/WT:_Flt3_ITD/WT mice. (E) Copy number ratio of _Mll_-WT:_Mll_-PTD was calculated for age-matched nonleukemic 15- to 25-week-old _Mll_PTD/WT:_Flt3_ITD/WT and leukemic _Mll_PTD/WT:_Flt3_ITD/ITD mice. Results are representative of 2 age-matched cohorts of mice.
Similar articles
- Comparative analysis of MLL partial tandem duplication and FLT3 internal tandem duplication mutations in 956 adult patients with acute myeloid leukemia.
Steudel C, Wermke M, Schaich M, Schäkel U, Illmer T, Ehninger G, Thiede C. Steudel C, et al. Genes Chromosomes Cancer. 2003 Jul;37(3):237-51. doi: 10.1002/gcc.10219. Genes Chromosomes Cancer. 2003. PMID: 12759922 - Eradicating acute myeloid leukemia in a Mll(PTD/wt):Flt3(ITD/wt) murine model: a path to novel therapeutic approaches for human disease.
Bernot KM, Nemer JS, Santhanam R, Liu S, Zorko NA, Whitman SP, Dickerson KE, Zhang M, Yang X, McConnell KK, Ahmed EH, Muñoz MR, Siebenaler RF, Marcucci GG, Mundy-Bosse BL, Brook DL, Garman S, Dorrance AM, Zhang X, Zhang J, Lee RJ, Blum W, Caligiuri MA, Marcucci G. Bernot KM, et al. Blood. 2013 Nov 28;122(23):3778-83. doi: 10.1182/blood-2013-06-507426. Epub 2013 Oct 1. Blood. 2013. PMID: 24085765 Free PMC article. - FLT3-ITD and MLL-PTD influence the expression of MDR-1, MRP-1, and BCRP mRNA but not LRP mRNA assessed with RQ-PCR method in adult acute myeloid leukemia.
Nasilowska-Adamska B, Solarska I, Paluszewska M, Malinowska I, Jedrzejczak WW, Warzocha K. Nasilowska-Adamska B, et al. Ann Hematol. 2014 Apr;93(4):577-93. doi: 10.1007/s00277-013-1898-7. Epub 2013 Sep 13. Ann Hematol. 2014. PMID: 24030729 Clinical Trial. - The MLL partial tandem duplication in acute myeloid leukaemia.
Basecke J, Whelan JT, Griesinger F, Bertrand FE. Basecke J, et al. Br J Haematol. 2006 Nov;135(4):438-49. doi: 10.1111/j.1365-2141.2006.06301.x. Epub 2006 Sep 11. Br J Haematol. 2006. PMID: 16965385 Review. - Prognostic implications of gene mutations in acute myeloid leukemia with normal cytogenetics.
Gaidzik V, Döhner K. Gaidzik V, et al. Semin Oncol. 2008 Aug;35(4):346-55. doi: 10.1053/j.seminoncol.2008.04.005. Semin Oncol. 2008. PMID: 18692685 Review.
Cited by
- E3 ubiquitin ligase Cbl-b activates the p53 pathway by targeting Siva1, a negative regulator of ARF, in FLT3 inhibitor-resistant acute myeloid leukemia.
Park IK, Blum W, Baker SD, Caligiuri MA. Park IK, et al. Leukemia. 2017 Feb;31(2):502-505. doi: 10.1038/leu.2016.293. Epub 2016 Oct 24. Leukemia. 2017. PMID: 27773928 Free PMC article. No abstract available. - DNMT3A mutants provide proliferating advantage with augmentation of self-renewal activity in the pathogenesis of AML in KMT2A-PTD-positive leukemic cells.
Bera R, Chiu MC, Huang YJ, Huang G, Lee YS, Shih LY. Bera R, et al. Oncogenesis. 2020 Feb 3;9(2):7. doi: 10.1038/s41389-020-0191-6. Oncogenesis. 2020. PMID: 32015320 Free PMC article. - Direct and Indirect Targeting of HOXA9 Transcription Factor in Acute Myeloid Leukemia.
Lambert M, Alioui M, Jambon S, Depauw S, Van Seuningen I, David-Cordonnier MH. Lambert M, et al. Cancers (Basel). 2019 Jun 17;11(6):837. doi: 10.3390/cancers11060837. Cancers (Basel). 2019. PMID: 31213012 Free PMC article. Review. - Identification of a germline F692L drug resistance variant in cis with Flt3-internal tandem duplication in knock-in mice.
Dovey OM, Chen B, Mupo A, Friedrich M, Grove CS, Cooper JL, Lee B, Varela I, Huang Y, Vassiliou GS. Dovey OM, et al. Haematologica. 2016 Aug;101(8):e328-31. doi: 10.3324/haematol.2016.146159. Epub 2016 May 12. Haematologica. 2016. PMID: 27175030 Free PMC article. No abstract available. - MicroRNA networks in FLT3-ITD acute myeloid leukemia.
Hoang DH, Zhao D, Branciamore S, Maestrini D, Rodriguez IR, Kuo YH, Rockne R, Khaled SK, Zhang B, Nguyen LXT, Marcucci G. Hoang DH, et al. Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2112482119. doi: 10.1073/pnas.2112482119. Epub 2022 Apr 11. Proc Natl Acad Sci U S A. 2022. PMID: 35412895 Free PMC article.
References
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7(11):823–833. - PubMed
- Caligiuri MA, Schichman SA, Strout MP, et al. Molecular rearrangement of the ALL-1 gene in acute myeloid leukemia without cytogenetic evidence of 11q23 chromosomal translocations. Cancer Res. 1994;54(2):370–373. - PubMed
- Steudel C, Wermke M, Schaich M, et al. Comparative analysis of MLL partial tandem duplication and FLT3 internal tandem duplication mutations in 956 adult patients with acute myeloid leukemia. Genes Chromosomes Cancer. 2003;37(3):237–251. - PubMed
- Shih LY, Liang DC, Fu JF, et al. Characterization of fusion partner genes in 114 patients with de novo acute myeloid leukemia and MLL rearrangement. Leukemia. 2006;20(2):218–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM12453/GM/NIGMS NIH HHS/United States
- CA089341/CA/NCI NIH HHS/United States
- R01 CA089341/CA/NCI NIH HHS/United States
- CA009338/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- T32 CA009338/CA/NCI NIH HHS/United States
- K08 CA113434/CA/NCI NIH HHS/United States
- P50 CA140158/CA/NCI NIH HHS/United States
- R01 CA102031/CA/NCI NIH HHS/United States
- CA140158/CA/NCI NIH HHS/United States
- CA113434/CA/NCI NIH HHS/United States
- CA102031/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous