ERLIN2 promotes breast cancer cell survival by modulating endoplasmic reticulum stress pathways - PubMed (original) (raw)
ERLIN2 promotes breast cancer cell survival by modulating endoplasmic reticulum stress pathways
Guohui Wang et al. BMC Cancer. 2012.
Abstract
Background: Amplification of the 8p11-12 region has been found in approximately 15% of human breast cancer and is associated with poor prognosis. Previous genomic analysis has led us to identify the endoplasmic reticulum (ER) lipid raft-associated 2 (ERLIN2) gene as one of the candidate oncogenes within the 8p11-12 amplicon in human breast cancer, particularly in the luminal subtype. ERLIN2, an ER membrane protein, has recently been identified as a novel mediator of ER-associated degradation. Yet, the biological roles of ERLIN2 and molecular mechanisms by which ERLIN2 coordinates ER pathways in breast carcinogenesis remain unclear.
Methods: We established the MCF10A-ERLIN2 cell line, which stably over expresses ERLIN2 in human nontransformed mammary epithelial cells (MCF10A) using the pLenti6/V5-ERLIN2 construct. ERLIN2 over expressing cells and their respective parental cell lines were assayed for in vitro transforming phenotypes. Next, we knocked down the ERLIN2 as well as the ER stress sensor IRE1α activity in the breast cancer cell lines to characterize the biological roles and molecular basis of the ERLIN2 in carcinogenesis. Finally, immunohistochemical staining was performed to detect ERLIN2 expression in normal and cancerous human breast tissues
Results: We found that amplification of the ERLIN2 gene and over expression of the ERLIN2 protein occurs in both luminal and Her2 subtypes of breast cancer. Gain- and loss-of-function approaches demonstrated that ERLIN2 is a novel oncogenic factor associated with the ER stress response pathway. The IRE1α/XBP1 axis in the ER stress pathway modulated expression of ERLIN2 protein levels in breast cancer cells. We also showed that over expression of ERLIN2 facilitated the adaptation of breast epithelial cells to ER stress by supporting cell growth and protecting the cells from ER stress-induced cell death.
Conclusions: ERLIN2 may confer a selective growth advantage for breast cancer cells by facilitating a cytoprotective response to various cellular stresses associated with oncogenesis. The information provided here sheds new light on the mechanism of breast cancer malignancy.
Figures
Figure 1
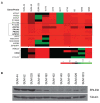
(a) Genomic copy number profiles of the ERLIN2 region analyzed on the Agilent oligonucleotide array CGH in 3 SUM breast cancer cell lines and 7 primary breast cancer specimens. Tumors are displayed vertically and array probes are displayed horizontally by genome position. Log2 ratio in a single sample is relative to normal female DNA and is depicted according to the color scale (bottom). Red indicates relative copy number gain, whereas green indicates relative copy number loss. (b) ERLIN2 protein levels were analyzed by Western blot in ten breast cancer cell lines with or without ERLIN2 amplification.
Figure 2
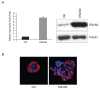
(a) Stable overexpressing ERLIN2 in MCF10A cells (MCF10A-ERLIN2) with the pLenti6/V5-ERLIN2 construct. Over expression of ERLIN2 mRNA and protein in this cell line was confirmed with semi-quantitative RT-PCR (right panel) and western blot assays (left panel). (b) Effects of ERLIN2 on mammary acinar morphogenesis. MCF10A-ERLIN2 and control cells were cultured on a bed of Matrigel. Representative images show the structures with staining for actin with phalloidin conjugated to Alexa Fluor-568 (red), and DAPI as a marker of nuclei (blue).
Figure 3
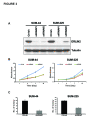
shRNA-mediated knockdown of ERLIN2 inhibits monolayer and anchorage-independent cell growth in breast cancer cells with ERLIN2 amplification. (a) Knockdown of ERLIN2 expression in SUM-44 and SUM-225 cells with two different shRNAs was confirmed by western blot. (b) In vitro growth rate of SUM-44 and SUM-225 cells with ERLIN2 shRNA treatment compared to cells with control shRNA treatment. (c) Knockdown of ERLIN2 reduces colony formation in soft agar. SUM-44 and SUM-225 cells were tranfected with ERLIN2 shRNA#1 or control shRNA. The colony numbers were counted 3 weeks later (P < 0.05).
Figure 4
(a) The knockdown of the IRE1α RNase activity (K907A) reduced levels of ERLIN2 protein in SUM-44 cells. Forced expression of wild-type IRE1α (b) and its substrate, spliced XBP1 (c), leads to increased expression of ERLIN2 at protein level in MCF10A cells. (d) ERLIN2 expression in MCF10A cells was analyzed by western blot after culture 48 hours in insulin- or EGF-depleted media, compared to that in normal culture media.
Figure 5
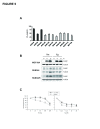
(a) IC 50 values for the ER stress-inducing reagent Tm, in ten breast cancer cell lines as well as in the MCF10A cells (b) The expression level of CHOP in SUM-225, SUM-44 breast cancer cells and MCF10A control cells was analyzed with Western blot after Tm (500 ng) or Tg (400 nM) treatment. (c) Cell viability of ERLIN2 knockdown and control SUM-225 cells was measured with MTT assays after exposure to different concentrations of the Tm or Tg for 72 hours.
Figure 6
Immunohistotochemical staining of ERLIN2 protein on a representative HBC sample and a normal control.
Similar articles
- MiR-410 Acts as a Tumor Suppressor in Estrogen Receptor-Positive Breast Cancer Cells by Directly Targeting ERLIN2 via the ERS Pathway.
Wu H, Li J, Guo E, Luo S, Wang G. Wu H, et al. Cell Physiol Biochem. 2018;48(2):461-474. doi: 10.1159/000491777. Epub 2018 Jul 17. Cell Physiol Biochem. 2018. PMID: 30016800 - TMEM33: a new stress-inducible endoplasmic reticulum transmembrane protein and modulator of the unfolded protein response signaling.
Sakabe I, Hu R, Jin L, Clarke R, Kasid UN. Sakabe I, et al. Breast Cancer Res Treat. 2015 Sep;153(2):285-97. doi: 10.1007/s10549-015-3536-7. Epub 2015 Aug 13. Breast Cancer Res Treat. 2015. PMID: 26268696 Free PMC article. - Endoplasmic reticulum factor ERLIN2 regulates cytosolic lipid content in cancer cells.
Wang G, Zhang X, Lee JS, Wang X, Yang ZQ, Zhang K. Wang G, et al. Biochem J. 2012 Sep 15;446(3):415-25. doi: 10.1042/BJ20112050. Biochem J. 2012. PMID: 22690709 Free PMC article. - Targeting the IRE1α-XBP1 branch of the unfolded protein response in human diseases.
Jiang D, Niwa M, Koong AC. Jiang D, et al. Semin Cancer Biol. 2015 Aug;33:48-56. doi: 10.1016/j.semcancer.2015.04.010. Epub 2015 May 16. Semin Cancer Biol. 2015. PMID: 25986851 Free PMC article. Review. - Endoplasmic reticulum quality control in cancer: Friend or foe.
Kim H, Bhattacharya A, Qi L. Kim H, et al. Semin Cancer Biol. 2015 Aug;33:25-33. doi: 10.1016/j.semcancer.2015.02.003. Epub 2015 Mar 18. Semin Cancer Biol. 2015. PMID: 25794824 Free PMC article. Review.
Cited by
- Eukaryotic initiation factor 4E-binding protein as an oncogene in breast cancer.
Rutkovsky AC, Yeh ES, Guest ST, Findlay VJ, Muise-Helmericks RC, Armeson K, Ethier SP. Rutkovsky AC, et al. BMC Cancer. 2019 May 23;19(1):491. doi: 10.1186/s12885-019-5667-4. BMC Cancer. 2019. PMID: 31122207 Free PMC article. - Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms.
Fink JK. Fink JK. Acta Neuropathol. 2013 Sep;126(3):307-28. doi: 10.1007/s00401-013-1115-8. Epub 2013 Jul 30. Acta Neuropathol. 2013. PMID: 23897027 Free PMC article. Review. - Amplification of WHSC1L1 regulates expression and estrogen-independent activation of ERα in SUM-44 breast cancer cells and is associated with ERα over-expression in breast cancer.
Irish JC, Mills JN, Turner-Ivey B, Wilson RC, Guest ST, Rutkovsky A, Dombkowski A, Kappler CS, Hardiman G, Ethier SP. Irish JC, et al. Mol Oncol. 2016 Jun;10(6):850-65. doi: 10.1016/j.molonc.2016.02.003. Epub 2016 Feb 27. Mol Oncol. 2016. PMID: 27005559 Free PMC article. - SNHG17 drives malignant behaviors in astrocytoma by targeting miR-876-5p/ERLIN2 axis.
Du F, Hou Q. Du F, et al. BMC Cancer. 2020 Sep 3;20(1):839. doi: 10.1186/s12885-020-07280-8. BMC Cancer. 2020. PMID: 32883232 Free PMC article. - Endoplasmic reticulum stress-related genes as prognostic and immunogenic biomarkers in prostate cancer.
Wan L, Fan Y, Wu T, Liu Y, Zhang R, Chen S, Zhao C, Xue Y. Wan L, et al. Eur J Med Res. 2024 Apr 20;29(1):242. doi: 10.1186/s40001-024-01818-3. Eur J Med Res. 2024. PMID: 38643190 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 1R21ES017829-01A1/ES/NIEHS NIH HHS/United States
- P30 ES06639/ES/NIEHS NIH HHS/United States
- P30-CA022453-29/CA/NCI NIH HHS/United States
- R01 CA100724/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous