Identification and functional characterization of p130Cas as a substrate of protein tyrosine phosphatase nonreceptor 14 - PubMed (original) (raw)
Identification and functional characterization of p130Cas as a substrate of protein tyrosine phosphatase nonreceptor 14
P Zhang et al. Oncogene. 2013.
Abstract
Protein tyrosine phosphatase nonreceptor type 14 (PTPN14) is frequently mutated in a variety of human cancers. However, the cell signaling pathways regulated by PTPN14 largely remain to be elucidated. Here, we identify a list of potential substrates of PTPN14 using a phospho-proteomic approach. We show that p130 Crk-associated substrate (p130Cas) is a direct substrate of PTPN14 and that PTPN14 specifically regulates p130Cas phosphorylation at tyrosine residue 128 (Y128) in colorectal cancer (CRC) cells. We engineered CRC cells homozygous for a p130Cas Y128F knock-in mutant and found that these cells exhibit significantly reduced migration and colony formation, impaired anchorage-independent growth, slower xenograft tumor growth in nude mice and have decreased phosphorylation of AKT. Furthermore, we demonstrate that SRC phosphorylates p130Cas Y128 and that CRC cell lines harboring high levels of pY128Cas are more sensitive to SRC family kinase inhibitor Dasatinib. These findings suggest that p130Cas Y128 phosphorylation may be exploited as a predictive marker for Dasatinib response in cancer patients. In aggregate, our studies reveal a novel signaling pathway that has an important role in colorectal tumorigenesis.
Conflict of interest statement
Conflict of Interest:
The authors declare no conflict of interest.
Figures
Figure 1. Identification of potential substrates of PTPN14 using a phospho-proteomic approach
(A) Schematic diagram of a phospho-proteomic approach to identify potential substrates of PTPN14. Cell lysates were made from the parental SW480 CRC cells, stable clones expressing either a wild-type (WT) PTP domain or a PTPN14 D1079A trapping mutant PTP domain. Proteins were digested with trypsin and pY containing peptides were enriched by anti-pY antibody (pY100)-conjugated beads. Peptide mixtures were profiled by mass-spectrometry. (B) List of the top candidate substrates of PTPN14. ND, not detected.
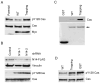
Figure 2. Validation of p130Cas as a substrate of PTPN14
(A) Overexpression of WT PTPN14 PTP domain decreases p130Cas Y128 phosphorylation, whereas the trapping mutant enriches pY128 Cas in CRC cells. Cell lysates made from the cell lines used in Fig. 1 were blotted with the indicated antibodies. (B) Knock-down of PTPN14 leads to increased levels of pY128 Cas in CRC cells. Cell lysates from stable clones of DLD1 CRC cells expressing scramble shRNAs or shRNAs against PTPN14 were blotted with the indicated antibodies. N14-1 and N14-2 are two independent shRNAs against PTPN14. (C) A PTPN14 trapping mutant traps p130Cas protein. GST, GST-WT and GST-D1079A trapping mutant PTPN14 PTP domains were expressed in E. Coli, purified and resolved on a SDS-PAGE gel (bottom panel). Arrow indicates the GST fusion proteins. Equal amounts of the purified protein were mixed with DLD1 cell lysates in the phosphatase trapping assay. The trapped p130Cas proteins were detected by Western blot (top panel). (D). PTPN14 dephosphorylates pY128 Cas in vitro. Phosphorylated p130Cas proteins were incubated with the indicated GST fusion proteins. The pY128 Cas proteins were quantified by Western blot analysis.
Figure 3. SRC phosphorylates the Y128 residue of p130Cas
(A) SRC kinase inhibitor treatment down-regulates pY128 p130Cas. DLD1 cells were treated with the indicated concentrations of SRC inhibitor AZD0530. Cell lysates were blotted with the indicated antibodies. (B) Overexpression of SRC results in increased levels of pY128 p130Cas. HEK 293 cells were transfected with either a Myc-tagged SRC plasmid or an empty vector. Cell lysates were blotted with the indicated antibodies. (C) Knock-down of SRC down-regulates pY128 Cas in CRC cells. Cell lysates from stable clones of DLD1 cells expressing scramble shRNAs or shRNAs against SRC were blotted with the indicated antibodies. SRC-1 and SRC-2 are two independent shRNAs against SRC. (D) CRC cells harboring high levels of pY128 Cas are more sensitive to SRC inhibitor Dasatinib-induced growth inhibition. The indicated CRC cell lines are treated with a serial concentration of Dasatinib. The IC50s of each cell line from three independent experiments are plotted. The levels of pY128 Cas in each cell line are quantified by Western blot analysis.
Figure 4. Generation of p130Cas Y128F mutant knockin (KI) CRC cells
(A) Diagram of the KI construct. (B) Genomic sequences of the parental (WT), heterozygous (Het) and homozygous (Hom) KI cells. The rectangular boxes indicate codons for the Cas amino acid 128 position. (C) The parental (WT) and p130Cas Y128F homozygous KI CRC cells were serum-starved and stimulated with EGF. Cell lysates were blotted with the indicated antibodies.
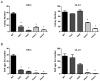
Figure 5. p130Cas Y128F mutant CRC cells are less tumorigenic in vitro
(A) p130Cas Y128F mutant CRC cells form fewer colonies. Cells from indicated clones were plated in 6-well plates in triplicates. Cells were grown for 14 days and stained with crystal violet. Colony numbers were counted and plotted for each of the clones. (B) p130Cas Y128F mutant CRC cells impair anchorage-independent growth. CRC cells of the indicated clones were mixed in 0.4% soft agar and plated in 6-well plates in triplicates. Cells were grown for 30 days. Colony foci were counted and plotted for each of the clones. * p < 0.0001, t test. Het and Hom indicate the heterozygous and homozygous KI clones respectively.
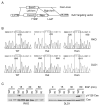
Figure 6. The RKO p130Cas Y128F mutant cells are less tumorigenic in vivo
Athymic nude mice were injected subcutaneously with cells from the indicated clones. Tumor sizes were measured weekly for 5 weeks. Mice were then sacrificed and tumors were harvested. (A) Tumors grown from the RKO clones. Each black rectangle indicates tumors harvested from a mouse. (B) Average sizes of the tumors formed by the indicated clones were plotted.
Figure 7. Reduced AKT phosphorylation in the p130Cas Y128F mutant cells
(A) and (B) Parental and p130Cas Y128F mutant (KI) cells were serum-starved and stimulated with EGF for the indicated time. Cell lysates were blotted with the indicated antibodies. (C) The p130Cas Y128F mutant proteins show weaker binding affinity to p85. Cell lysates from either the parental or p130Cas Y128F mutant (KI) cells were immunoprecipitated with an anti-p130Cas antibody and blotted with the indicated antibodies.
References
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001 May 17;411(6835):355–65. - PubMed
- Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004 May 21;304(5674):1164–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-CA127590/CA/NCI NIH HHS/United States
- P30 CA043703/CA/NCI NIH HHS/United States
- R01 HG004722/HG/NHGRI NIH HHS/United States
- R01-HG004722/HG/NHGRI NIH HHS/United States
- R01 CA127590/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous