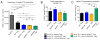Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms - PubMed (original) (raw)
Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms
Ulf H Beier et al. Sci Signal. 2012.
Abstract
Therapeutic inhibition of the histone deacetylases HDAC6, HDAC9, or sirtuin-1 (Sirt1) augments the suppressive functions of regulatory T cells (T(regs)) that contain the transcription factor Foxp3 (Forkhead box P3) and is useful in organ transplant patients or patients with autoimmune diseases. However, it is unclear whether distinct mechanisms are involved for each HDAC or whether combined inhibition of HDACs would be more effective. We compared the suppressive functions of T(regs) from wild-type C57BL/6 mice with those from mice with either complete or cell-specific deletion of various HDACs, as well as with those of T(regs) treated with isoform-selective HDAC inhibitors. The improvement of T(reg) suppressive function mediated by inhibition of HDAC6, but not Sirt1, required an intact heat shock response. Although HDAC6, HDAC9, and Sirt1 all deacetylated Foxp3, each protein had different effects on transcription factors that control expression of the gene encoding Foxp3. For example, loss of HDAC9, but not other HDACs, was associated with stabilization of the acetylated form of signal transducer and activator of transcription 5 (STAT5) and promoted its transcriptional activity. Thus, targeting different HDACs increased T(reg) function through multiple and additive mechanisms, which suggests the therapeutic potential for using combinations of HDAC inhibitors in the management of autoimmunity and organ transplantation.
Conflict of interest statement
Competing interests: None.
Figures
Fig. 1
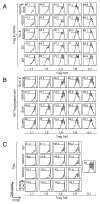
The heat shock response is required to improve Treg function in response to inhibition of HDAC6, but not Sirt1. (A) Increased Hsp90 K294 acetylation (aHsp90) in HDAC6−/− Tregs (H6) and HDAC9−/− Tregs (H9), and to a lesser degree in fl-Sirt1/CD4cre Tregs (SC). Densitometry data pooled from two experiments. Immunofluorescence of stimulated Tregs showed nuclear translocation of HSF1, mostly in HDAC6−/− cells, whereas fl-Sirt1/CD4cre Tregs exhibited the least amount of intranuclear HSF1. (B) Hsp70−/− Tregs had diminished suppressive function. Data are from three experiments. (C) Hsp70−/− Tregs were less responsive to ACY-738 (HDAC6i) than WT Tregs, especially under conditions of heat shock. Data are from three experiments. (D) Sirt1-deficient Tregs exhibited a trend to increased acetylation of Hsp90 compared to wild-type (WT) Tregs. Densitometry data pooled from two experiments. SF, fl-Sirt1/Foxp3cre. (E) Transcriptional repression of genes involved in the heat shock response in fl-Sirt1/CD4cre Tregs as analyzed by microarrays, with data shown after z-score transformation. (F) Apoptosis-free survival of Tregs after heat shock. (G) The suppressive function of Tregs was improved by inhibition of Sirt1 (with EX-527) in the absence of Hsp70 and under conditions of heat shock. Data are from four experiments. Effector T cells (Teff) and APCs used in the suppression assays were from (B) WT or (C and G) Hsp70−/− mice. WT refers to the appropriate control mouse strain, that is C57BL6 mice for (A), (D), (E), and (F) or B6/129 mice for (B), (C), and (G). *p<0.05, **p<0.01, and ***p<0.001. Scale bar = 10 μm. Additional statistics for (C) and (G) are available in tables S1 and S2.
Fig. 2
Functions of HDAC6 in Foxp3+ Tregs that are independent of the heat shock response. (A) Immunofluorescence showing the translocation of HDAC6 into the nuclei of WT Tregs after stimulation with antibodies against CD3ε and CD28. (B) Western blotting analysis of Treg lysates indicated that Foxp3 is more abundant in HDAC6−/− cells than in WT cells. Densitometry data pooled from of three experiments. (C) Co-immunoprecipitation of HDAC6 and Foxp3 in WT Treg lysates. (D) Immunoprecipitation studies of Treg lysates indicated that Foxp3 is more acetylated in HDAC6−/− Tregs than in WT Tregs. Densitometry data pooled from two experiments. (E) Proximity ligation assays showed more acetylated Foxp3 in HDAC6−/− Tregs than in WT Tregs. (F) Quantification of proximity ligation assays with BlobFinder and statistical analysis with the Kruskal-Wallis test. *P<0.05, ***P<0.001. Data shown as boxplot with the median, 25th, and 75th quartiles; whiskers indicate the 10th and 90th percentiles. Scalebar: 10 μm.
Fig. 3
Deletion of HDAC9 stabilizes the acetylation, phosphorylation, and transcriptional activity of STAT5. (A) Analysis of Treg lysates indicated that STAT5 was relatively more phosphorylated in HDAC9−/− cells than in WT cells. Densitometry data pooled from three experiments. (B) Conceptual model of the acetylation of STAT5, which protects it from losing its transcriptionally active, phosphorylated dimeric form. (C) Proximity ligation assay showed prominent acetylation of STAT5 in HDAC9−/− Tregs compared to that in WT Tregs. (D) Quantification of the data in (C) was performed as described for Fig. 2F. (E) Microarray analysis showed STAT5-dependent signaling in HDAC9−/− Tregs compared to WT Treg (n=3/group) based on previously reported STAT5 targets (19). (F) Further STAT5 signaling as well as other transcriptional alterations promoted a suppressive phenotype in HDAC9−/− Tregs. Data are shown after z-score transformation. *IL-10 not significant. Scale bar: 10 μm. Trp, transformation-related protein; Mcl, myeloid leukemia cell differentiating protein; Tnfsf, tumor necrosis factor superfamily; Id, inhibitor of DNA; Lta, lymphotoxin-α; Nos, NO synthase; Mdm2, an E3 ubiquitin ligase; Scl2a1, GLUT1; Trim, tripartite motif; Klk, Kallikrein; ptgs, post-transcriptional gene silencing; bmp2k, bone morphogenetic protein inducible kinase-2; Cd2ap, CD2-associated protein; Ikbke, Inhibitor of nuclear factor κB kinase ε; Iigp, IFN-inducible GTPase; Xiap, X-linked inhibitor of apoptosis; Fas, CD95; Bcl, B-cell lymphoma; mettl, methyltransferase-like; St8sia, ST8 α-N-acetyl-neuraminide α-2,8-sialyltransferase-4.
Fig. 4
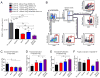
Combined targeting of HDACs can further augment the suppressive function of Tregs. (A) Treg suppression assays with WT effector T cells and APCs, as well as with WT or HDAC-deficient Tregs with or without one or two specific HDAC inhibitors. We used EX-527 (5 μM) and ACY-738 (1 μM) to inhibit Sirt1 and HDAC6, respectively. (B) WT Tregs treated with Sirt1i, HDCA6i, or both showed increased suppressive function compared to Tregs treated with single HDAC6i or Sirt1i, or vehicle control treatment. (C) HDAC6−/− HDAC9−/− double knockout Tregs showed stronger suppressive function compared with WT and single knockout Tregs. Data are representative of three independent experiments.
Fig. 5
Combined pharmacologic targeting of HDAC6 and Sirt1 is additive in improving the suppressive function of Tregs and preserving Foxp3 in vivo. (A) Homeostatic proliferation assays showed increased suppressive function of Tregs in mice after individual treatment with either Sirt1i (EX-527, 1 mg/kg/d i.p.) or HDAC6i (Tubastatin, 40 mg/kg/d i.p.) as well as further improvement with combined treatment. (B) Gating strategy to identify cell populations of interest in spleens harvested from B6/Rag1−/− mice; combined treatment sample shown. Red labeled boxes identify populations of cells subsequently analyzed in the indicated panels. (C) Ki67 is reduced in host non-CD4+ cells treated with Sirt1i or HDAC6i. (D) Foxp3+ was better preserved in Tregs from mice treated with Sirt1i and HDAC6i, especially in combination, than in untreated mice. (E) Tregs from mice treated with Sirt1i, HDAC6i, and combined treatment groups had a more activated phenotype (Ki67) than did untreated cells. (F) Neither Sirt1i nor HDAC6i improves the induction of Tregs from effector T cells. Data are from three mice per group (N=15). *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. 6
Combined genetic targeting of HDAC6 and HDAC9 does not substantially improve the suppressive function of Tregs in vivo. (A) Homeostatic proliferation assays showed improved suppression of effector T cells by Tregs with either individual deficiency in either HDAC6 or HDAC9, but no added benefit from combined deletion. (B) Preservation of Foxp3 was not changed by combined knockout of HDAC6 and HDAC9. (C) Ki67 abundance was increased in HDAC6−/− HDAC9−/− double knockout Treg compared to that in single knockout and WT cells. Data are from three to four mice per group (N=18). The gating strategy used was similar to that in Fig. 5B. *P < 0.05, **P < 0.01, and ***P <0.001.
Similar articles
- Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells.
de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, Hancock WW. de Zoeten EF, et al. Mol Cell Biol. 2011 May;31(10):2066-78. doi: 10.1128/MCB.05155-11. Epub 2011 Mar 28. Mol Cell Biol. 2011. PMID: 21444725 Free PMC article. - Histone/protein deacetylases control Foxp3 expression and the heat shock response of T-regulatory cells.
Beier UH, Akimova T, Liu Y, Wang L, Hancock WW. Beier UH, et al. Curr Opin Immunol. 2011 Oct;23(5):670-8. doi: 10.1016/j.coi.2011.07.002. Epub 2011 Jul 26. Curr Opin Immunol. 2011. PMID: 21798734 Free PMC article. Review. - Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice.
de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW. de Zoeten EF, et al. Gastroenterology. 2010 Feb;138(2):583-94. doi: 10.1053/j.gastro.2009.10.037. Epub 2009 Oct 29. Gastroenterology. 2010. PMID: 19879272 Free PMC article. - Combination of isoform-selective histone/protein deacetylase inhibitors improves Foxp3+ T-regulatory cell function.
Beier UH, Wang L, Hancock WW. Beier UH, et al. Cell Cycle. 2012 Sep 15;11(18):3351-2. doi: 10.4161/cc.21876. Epub 2012 Aug 23. Cell Cycle. 2012. PMID: 22918251 Free PMC article. - Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance.
Wang L, Tao R, Hancock WW. Wang L, et al. Immunol Cell Biol. 2009 Mar-Apr;87(3):195-202. doi: 10.1038/icb.2008.106. Epub 2009 Jan 27. Immunol Cell Biol. 2009. PMID: 19172156 Review.
Cited by
- Butyrate as a bioactive human milk protective component against food allergy.
Paparo L, Nocerino R, Ciaglia E, Di Scala C, De Caro C, Russo R, Trinchese G, Aitoro R, Amoroso A, Bruno C, Di Costanzo M, Passariello A, Messina F, Agangi A, Napolitano M, Voto L, Gatta GD, Pisapia L, Montella F, Mollica MP, Calignano A, Puca A, Berni Canani R. Paparo L, et al. Allergy. 2021 May;76(5):1398-1415. doi: 10.1111/all.14625. Epub 2020 Nov 16. Allergy. 2021. PMID: 33043467 Free PMC article. - Role of Histone Deacetylase 6 and Histone Deacetylase 6 Inhibition in Colorectal Cancer.
Vuletić A, Mirjačić Martinović K, Spasić J. Vuletić A, et al. Pharmaceutics. 2023 Dec 29;16(1):54. doi: 10.3390/pharmaceutics16010054. Pharmaceutics. 2023. PMID: 38258065 Free PMC article. Review. - Sirt-ainly Aire.
Peterson P. Peterson P. Nat Immunol. 2015 Jul;16(7):680-1. doi: 10.1038/ni.3195. Nat Immunol. 2015. PMID: 26086133 No abstract available. - Metabolic Control of Treg Cell Stability, Plasticity, and Tissue-Specific Heterogeneity.
Shi H, Chi H. Shi H, et al. Front Immunol. 2019 Dec 11;10:2716. doi: 10.3389/fimmu.2019.02716. eCollection 2019. Front Immunol. 2019. PMID: 31921097 Free PMC article. Review. - Commensal microbiota regulates T cell fate decision in the gut.
Furusawa Y, Obata Y, Hase K. Furusawa Y, et al. Semin Immunopathol. 2015 Jan;37(1):17-25. doi: 10.1007/s00281-014-0455-3. Epub 2014 Oct 15. Semin Immunopathol. 2015. PMID: 25315350 Review.
References
- Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. - PubMed
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. - PubMed
- Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. - PubMed
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08AI095353/AI/NIAID NIH HHS/United States
- R56 AI095276/AI/NIAID NIH HHS/United States
- AI095276/AI/NIAID NIH HHS/United States
- AI073489/AI/NIAID NIH HHS/United States
- P01 AI073489/AI/NIAID NIH HHS/United States
- K08 AI095353/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous