PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity - PubMed (original) (raw)
PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity
Gerald R V Hammond et al. Science. 2012.
Abstract
The quantitatively minor phospholipid phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P(2)] fulfills many cellular functions in the plasma membrane (PM), whereas its synthetic precursor, phosphatidylinositol 4-phosphate (PI4P), has no assigned PM roles apart from PI(4,5)P(2) synthesis. We used a combination of pharmacological and chemical genetic approaches to probe the function of PM PI4P, most of which was not required for the synthesis or functions of PI(4,5)P(2). However, depletion of both lipids was required to prevent PM targeting of proteins that interact with acidic lipids or activation of the transient receptor potential vanilloid 1 cation channel. Therefore, PI4P contributes to the pool of polyanionic lipids that define plasma membrane identity and to some functions previously attributed specifically to PI(4,5)P(2), which may be fulfilled by a more general polyanionic lipid requirement.
Figures
Fig. 1
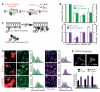
Independent depletion of PM PI4P and PI(4,5)P2. (A) Synthesis of PI(4,5)P2, and effects of inhibitors/activators. (B) Effect of LY294002, PAO and ionomycin on PI4P and PI(4,5)P2 measured by mass spectrometry (open bars) or staining (filled bars; means ± SEM, n = 3-4). (C) Generation of Pseudojanin (PJ), a fusion of sac and INPP5E phosphatase domains with FKBP, and its rapamycin-induced recruitment to a PM targeted FRB domain (Lyn11-FRB). (D) Effect of PJ, PJ-Sac (with inactivated INPP5E domain) or INPP5E (lacking the sac domain) on PI4P and PI(4,5)P2 staining intensity after PM recruitment for 2 min with 1 μM rapamycin. Histograms are means ± SEM (n = 4-5); gray peaks are the frequency of occurrence of cells with the indicated staining intensity for mock-transfected cells. (E) Effect of PJ constructs on PM recruitment of PI4P/PI(4,5)P2-binding GFP-PH-Osh2x2 (example images) and the PI(4,5)P2-selective PH-PLCδ1 and Tubbyc domains (means ± SEM of 10-18 cells).
Fig. 2
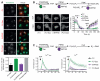
Dependence of clathrin-mediated endocytosis, PI3K and PLC signaling on PM PI(4,5)P2, but not PI4P. (A) Effect of PM-recruited PJ constructs (red) on the uptake of transferrin (AlexaFluor®488, 20 μg/ml, green) over 15 min prior to removing surface bound transferrin with a pH 2.5 wash. The bar chart shows the proportion of cells showing uptake of transferrin-associated fluorescence. Data are means ± SEM (n = 4). (B) Effect of PM recruitment of PJ constructs on the PI(3,4)P2/PI(3,4,5)P3 reporter GFP-PH-Akt after stimulation of serum starved cells for 2 min with IGF-1. Data are means ± SEM from 15-26 cells. (C) Effect of PM-recruited PJ constructs on PLC-mediated Ca2+ signals (monitored with Ca2+ indicator Fluo4-AM) after stimulation of endogenous P2Y-receptors with 100 μM ATP in either calcium-free (100 μM EGTA) or Ca2+-containing (1.8 mM) medium. Data are means ± SEM from 11-26 cells.
Fig. 3
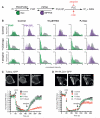
Requirements of PI4K activity but not PM PI4P for re-synthesis of PI(4,5)P2 after robust PLC activation. (A) In muscarinic M1 receptor expressing cells, PLC activity is stimulated with carbachol (CCh) and inactivated by atropine. (B) Effect of PAO or PJ-Sac (recruited to the PM with rapamycin) on PI4P and PI(4,5)P2 staining before, after 2 min 1mM CCh stimulation or after a further 3 min of 10 μM atropine treatment. Histograms are the relative frequency of occurrence of PI4P and PI(4,5)P2 staining intensities expressed as means ± SEM (n = 4). Gray peaks are data from control, un-stimulated cells. (C and D) Effect of PAO or PJ-Sac recruited to the PM on PI(4,5)P2 re-synthesis assayed with the PI(4,5)P2 biosensors Tubbyc-GFP (C) or PH-PLCδ1-GFP (D) during stimulation with CCh and subsequent inhibition with atropine. Data are means ± SEM of 8-26 cells. Images show cells co-expressing the reporters with Lyn11-FRB-CFP and PJ-Sac.
Fig. 4
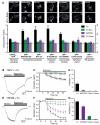
(A) Targeting of proteins with polybasic motifs to the PM by PI4P and PI(4,5)P2. Representative images before and after rapamycin treatment, and dissociation index of cells transfected with the indicated GFP-tagged motifs, Lyn11-FRB-CFP and the indicated PJ construct. Data are means ± SEM for 8-19 cells. (B-D) Capsaicin-induced currents in HEK293 cells expressing TRPV1 are enabled by the presence of either PI4P or PI(4,5)P2. (B) Specimen showing rapamycin induced inactivation in the presence of Pseudojanin. (C) Time course of the capsaicin-induced current in the presence of Pseudojanin, INPP5E, PJ-Sac or PJ-Dead. (D) Percentage of inhibition at the end of the 90 s co-application of rapamycin 1 μM (n = 8). (E-G) Menthol-induced currents in HEK293 expressing TRPM8 depend primarily on the presence of PI(4,5)P2. (E) Representative specimen showing the effect of Pseudojanin. (F) Time course of the menthol-induced current in the presence of Pseudojanin, INPP5E, PJ-Sac or PJ-Dead. (G) Percentage of inhibition at the end of the 90 s co-application of rapamycin 1 μM (n = 7).
Comment in
- Cell biology. Precursor or charge supplier?
Fairn GD, Grinstein S. Fairn GD, et al. Science. 2012 Aug 10;337(6095):653-4. doi: 10.1126/science.1227096. Science. 2012. PMID: 22879491 No abstract available.
Similar articles
- Nanoscale Landscape of Phosphoinositides Revealed by Specific Pleckstrin Homology (PH) Domains Using Single-molecule Superresolution Imaging in the Plasma Membrane.
Ji C, Zhang Y, Xu P, Xu T, Lou X. Ji C, et al. J Biol Chem. 2015 Nov 6;290(45):26978-26993. doi: 10.1074/jbc.M115.663013. Epub 2015 Sep 22. J Biol Chem. 2015. PMID: 26396197 Free PMC article. - PI(4,5)P2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER-PM contact sites.
Sohn M, Korzeniowski M, Zewe JP, Wills RC, Hammond GRV, Humpolickova J, Vrzal L, Chalupska D, Veverka V, Fairn GD, Boura E, Balla T. Sohn M, et al. J Cell Biol. 2018 May 7;217(5):1797-1813. doi: 10.1083/jcb.201710095. Epub 2018 Feb 22. J Cell Biol. 2018. PMID: 29472386 Free PMC article. - Differential Regulation of Ca2+-Activated Cl- Channel TMEM16A Splice Variants by Membrane PI(4,5)P2.
Ko W, Suh BC. Ko W, et al. Int J Mol Sci. 2021 Apr 15;22(8):4088. doi: 10.3390/ijms22084088. Int J Mol Sci. 2021. PMID: 33920953 Free PMC article. - Phosphatidylinositol 4-phosphate: a key determinant of plasma membrane identity and function in plants.
Marković V, Jaillais Y. Marković V, et al. New Phytol. 2022 Aug;235(3):867-874. doi: 10.1111/nph.18258. Epub 2022 Jun 8. New Phytol. 2022. PMID: 35586972 Review. - Regulation of yeast polarized exocytosis by phosphoinositide lipids.
Volpiana MW, Nenadic A, Beh CT. Volpiana MW, et al. Cell Mol Life Sci. 2024 Nov 19;81(1):457. doi: 10.1007/s00018-024-05483-x. Cell Mol Life Sci. 2024. PMID: 39560727 Free PMC article. Review.
Cited by
- Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons.
Lukacs V, Yudin Y, Hammond GR, Sharma E, Fukami K, Rohacs T. Lukacs V, et al. J Neurosci. 2013 Jul 10;33(28):11451-63. doi: 10.1523/JNEUROSCI.5637-12.2013. J Neurosci. 2013. PMID: 23843517 Free PMC article. - Phosphatidylinositol 4,5-Bisphosphate Modulates the Affinity of Talin-1 for Phospholipid Bilayers and Activates Its Autoinhibited Form.
Ye X, McLean MA, Sligar SG. Ye X, et al. Biochemistry. 2016 Sep 13;55(36):5038-48. doi: 10.1021/acs.biochem.6b00497. Epub 2016 Aug 31. Biochemistry. 2016. PMID: 27548281 Free PMC article. - Orthogonal Targeting of SAC1 to Mitochondria Implicates ORP2 as a Major Player in PM PI4P Turnover.
Doyle CP, Rectenwald A, Timple L, Hammond GRV. Doyle CP, et al. Contact (Thousand Oaks). 2024 Feb 7;7:25152564241229272. doi: 10.1177/25152564241229272. eCollection 2024 Jan-Dec. Contact (Thousand Oaks). 2024. PMID: 38327560 Free PMC article. - Regulation of phosphatidylinositol-specific phospholipase C at the nuclear envelope in cardiac myocytes.
Smrcka AV. Smrcka AV. J Cardiovasc Pharmacol. 2015 Mar;65(3):203-10. doi: 10.1097/FJC.0000000000000195. J Cardiovasc Pharmacol. 2015. PMID: 25658460 Free PMC article. Review. - Characterization of the c10orf76-PI4KB complex and its necessity for Golgi PI4P levels and enterovirus replication.
McPhail JA, Lyoo H, Pemberton JG, Hoffmann RM, van Elst W, Strating JRPM, Jenkins ML, Stariha JTB, Powell CJ, Boulanger MJ, Balla T, van Kuppeveld FJM, Burke JE. McPhail JA, et al. EMBO Rep. 2020 Feb 5;21(2):e48441. doi: 10.15252/embr.201948441. Epub 2019 Dec 12. EMBO Rep. 2020. PMID: 31829496 Free PMC article.
References
- Suh B-C, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005;15:370–378. - PubMed
- Di Paolo G, de Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. - PubMed
- McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- BBS/E/B/000C0415/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- ZIA HD000196/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous