Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson's disease - PubMed (original) (raw)
Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson's disease
Avik Roy et al. PLoS One. 2012.
Abstract
Neuroinflammation and oxidative stress underlie the pathogenesis of various neurodegenerative disorders. Here we demonstrate that sodium phenylbutyrate (NaPB), an FDA-approved therapy for reducing plasma ammonia and glutamine in urea cycle disorders, can suppress both proinflammatory molecules and reactive oxygen species (ROS) in activated glial cells. Interestingly, NaPB also decreased the level of cholesterol but involved only intermediates, not the end product of cholesterol biosynthesis pathway for these functions. While inhibitors of both geranylgeranyl transferase (GGTI) and farnesyl transferase (FTI) inhibited the activation of NF-κB, inhibitor of GGTI, but not FTI, suppressed the production of ROS. Accordingly, a dominant-negative mutant of p21(rac), but not p21(ras), attenuated the production of ROS from activated microglia. Inhibition of both p21(ras) and p21(rac) activation by NaPB in microglial cells suggests that NaPB exerts anti-inflammatory and antioxidative effects via inhibition of these small G proteins. Consistently, we found activation of both p21(ras) and p21(rac)in vivo in the substantia nigra of acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Oral administration of NaPB reduced nigral activation of p21(ras) and p21(rac), protected nigral reduced glutathione, attenuated nigral activation of NF-κB, inhibited nigral expression of proinflammatory molecules, and suppressed nigral activation of glial cells. These findings paralleled dopaminergic neuronal protection, normalized striatal neurotransmitters, and improved motor functions in MPTP-intoxicated mice. Consistently, FTI and GGTI also protected nigrostriata in MPTP-intoxicated mice. Furthermore, NaPB also halted the disease progression in a chronic MPTP mouse model. These results identify novel mode of action of NaPB and suggest that NaPB may be of therapeutic benefit for neurodegenerative disorders.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
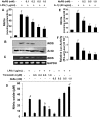
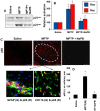
Figure 1. Dose-dependent inhibition of NO production by NaPB in mouse and human glial cells.
Primary mouse microglia were treated with different concentrations of NaPB for 6 h followed by stimulation with LPS under serum-free condition. After 24 h of stimulation, concentrations of nitrite were measured in supernatants (A) and the level of iNOS protein was monitored in cells by Western blot (B). Results are mean
SD of three different experiments. ap<0.001 vs control; bp<0.05 vs LPS; cp<0.001 vs LPS. After 5 h of stimulation, the expression of iNOS mRNA was monitored by semi-quantitative RT-PCR (C). Cells preincubated with different concentration of trichostatin A (TSA) and sodium butyrate (NaBu) for 6 h were stimulated with LPS for 24 h under serum-free condition followed by monitoring the level of nitrite in supernatants (D). Results are mean
S.D. of three different experiments. ap<0.001 vs control; bp<0.05 vs LPS; c,dp<0.001 vs LPS. Primary human astroglia isolated from fetal brain tissues were treated with different concentrations of NaPB for 6 h followed by stimulation with IL-1β under serum-free condition. After 48 h of stimulation, concentrations of nitrite (E) were measured in supernatants. Human astroglia plated at 70–80% confluence in 12-well plates were cotransfected with 0.25 µg of phiNOS(7.2)Luc and 12.5 ng of pRL-TK using the Lipofectamine-Plus (Invitrogen). Twenty-four h after transfection, cells received NaPB. After 6 h of incubation, cells were stimulated with IL-1β (20 ng/ml) for 12 h. Firefly (ff-Luc) and Renilla (r-Luc) luciferase activities were obtained by analyzing the total cell extract (F). Data are mean
S.D. of three different experiments. ap<0.001 versus control; bp<0.05 versus IL-1β; cp<0.001 versus IL-1β.
Figure 2. NaPB attenuates activation of NF-κB in mouse microglial cells.
(A) BV-2 microglial cells preincubated with 0.5 mM NaPB for 6 h were stimulated with 1 µg/ml LPS. At different minute of stimulation, the level of phospho-IκBα was monitored by Western blot. B) Cells preincubated with different concentrations of NaPB for 6 h were stimulated with LPS for 1 h followed by monitoring the DNA-binding activity of NF-κB by EMSA. (C) Cells plated in 12-well plates were co-transfected with 0.25 µg of PBIIX-Luc (an NF-κB-dependent reporter construct) and 12.5 ng of pRL-TK. Twenty-four h after transfection, cells received different concentrations of NaPB. After 6 h of incubation, cells were stimulated with LPS for 4 h. Firefly (ff-Luc) and Renilla (r-Luc) luciferase activities were obtained by analyzing the total cell extract. Results are mean
S.D. of three different experiments. ap<0.001 vs control; bp<0.05 vs LPS; cp<0.001 vs LPS. D) Cells preincubated with 0.5 mM NaPB for 6 h were stimulated with LPS for 2 h followed by monitoring the recruitment of RelA p65 to the mouse iNOS promoter by ChIP assay. E) Cells were co-transfected with 0.25 µg of PBIIX-Luc and 12.5 ng of pRL-TK. Twenty-four hours after transfection, cells were incubated with NaPB in the presence or absence of HMG-CoA, mevalonate, FPP, GGPP, cholesterol, and coenzyme Q. After 6 h of incubation, cells were stimulated with LPS for 4 h followed by assay of firefly (ff-Luc) and Renilla (r-Luc) luciferase activities. Results are mean
S.D. of three different experiments. ap<0.001 vs control; bp<0.001 vs LPS; cp<0.001 vs LPS+NaPB.
Figure 3. NaPB inhibits the production of ROS in mouse microglial cells.
Cells were treated with 500 µM NaPB for 6 h followed by stimulation with 1 µM MPP+. At 15 min of stimulation, the generation of ROS was monitored by carboxy-H2DCFDA (A). At different intervals (measured in minutes), superoxide production was assayed in whole cells (B). Cells preincubated with 500 µM NaPB for 6 h were stimulated with LPS (1 µg/ml), TNF-α (50 ng/ml), IL-1β (20 ng/ml), gp120 (200 pg/ml), and fibrillar Aβ1-42 (1 µM). At 10 min of stimulation, superoxide production was assayed in whole cells (C). Results are mean
SD of three different experiments. ap<0.001 vs control; bp<0.001 vs stimuli. D) Cells were incubated with NaPB in the presence or absence of HMG-CoA, mevalonate, GGPP, FPP, cholesterol, and coenzyme Q. After 6 h of incubation, cells were stimulated with LPS for 10 min followed by assay of superoxide. Results are mean
SD of three different experiments. ap<0.001 vs control; bp<0.001 vs LPS; cp<0.001 vs LPS+NaPB.
Figure 4. NaPB inhibits the activation of NF-κB and the production of ROS via modulation of p21ras and p21rac.
A) Mouse BV-2 microglial cells preincubated with different concentrations of FTI and GGTI were stimulated with LPS for 60 min followed by monitoring the activation of NF-κB by EMSA. B) Cells preincubated with different concentrations of FTI and GGTI were stimulated with LPS for 10 min followed by monitoring the production of superoxide. Results are mean
SD of three different experiments. ap<0.001 vs control; bp<0.001 vs LPS. C) Cells were transfected with Δp21ras, Δp21rac or an empty vector using Lipofectamine Plus. Twenty-four hour after transfection, cells were stimulated with LPS followed by monitoring superoxide production at 15 and 30 min of stimulation. ap<0.001 vs control; bp<0.001 vs LPS-15 min; cp<0.001 vs LPS-30 min. D) Cells preincubated with 500 µM NaPB were stimulated with LPS. At different time points, activation of p21ras and p21rac was monitored. Results represent three independent experiments.
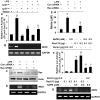
Figure 5. Role of p21ras and p21rac in the induction of iNOS and the activation of NF-κB in microglial cells.
A) Cells plated in 12-well plates were co-transfected with 0.2 µg PBIIX-Luc and 0.2 µg Δp21ras, Δp21rac or an empty vector. Each transfection also included 12.5 ng of pRL-TK. Twenty-four hour after transfection, cells were stimulated with LPS for 4 h. Firefly (ff-Luc) and Renilla (r-Luc) luciferase activities were obtained by analyzing the total cell extract. Results are mean
S.D. of three different experiments. ap<0.001 vs control; bp<0.001 vs LPS. B) Cells were transfected with Δp21ras, Δp21rac or an empty vector. Twenty-four hour after transfection, cells were stimulated with LPS for 5 h under serum-free condition followed by monitoring the expression of iNOS mRNA by RT-PCR. Cells were transfected with either control siRNA or Ras siRNA and 24 h after transfection, cells were stimulated with LPS for 5 h followed by monitoring the expression of Ras protein by Western blot (C) and iNOS mRNA by RT-PCR (D). After transfection, cells were stimulated with LPS for 24 h followed by monitoring the level of nitrite in supernatants (E). ap<0.001 vs control; bp<0.001 vs LPS. F) Cells plated in 12-well plates were co-transfected with 0.2 µg PBIIX-Luc and different amounts of either RasV12 (a constitutively-active mutant of p21ras) or control vector. Each transfection also included 12.5 ng of pRL-TK. Twenty-four hour after transfection, cells were treated with NaPB for 6 h under serum-free condition followed by monitoring firefly (ff-Luc) and Renilla (r-Luc) luciferase activities in total cell extract. G) Under similar condition, the effect of RasV12 and an empty vector on the expression of iNOS mRNA was monitored in cells. Results are mean
S.D. of three different experiments. ap<0.001 vs empty vector (0.2 µg); bp<0.001 vs RasV12 (0.1 µg); cp<0.001 vs RasV12 (0.2 µg).
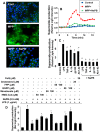
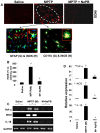
Figure 6. Activation of small G proteins (p21ras and p21rac) and NF-κB in ventral midbrain of MPTP-intoxicated mice is NaPB-sensitive.
A) Mice were treated with NaPB (200 mg/kg body wt/d) via gavage from 1 d prior to MPTP injection. Six h after the last injection of MPTP, activation of p21ras and p21rac was monitored in ventral midbrain tissues. Experiment was repeated three times each time using two animal in each group. B) Bands from three different mice were quantified and activation of p21ras and p21rac is shown as percent of control. C) Mice were treated with NaPB (200 mg/kg body wt/d) from 3 h after the last injection of MPTP. Twenty-four h after the last injection of MPTP, ventral midbrain sections were immunostained for p65 (low magnification). Midbrain sections of MPTP-intoxicated mice were also double-labeled for p65 and glial cell markers (GFAP for astrocytes and CD11b for microglia). Results represent three independent experiments. D) NF-κB p65 positive cells counted in four nigral sections (two images per slide) from each of four mice in an Olympus IX81 fluorescence microscope using the MicroSuite™ imaging software are mentioned as cells/mm2. ap<0.0001 vs saline-control; bp<0.0001 vs MPTP.
Figure 7. Expression of proinflammatory molecules in ventral midbrain of MPTP-intoxicated mice is NaPB-sensitive.
A) Mice were treated with NaPB (200 mg/kg body wt/d) from 3 h after the last injection of MPTP. Twenty-four h after the last injection of MPTP, ventral midbrain sections were immunostained for iNOS (low magnification). Midbrain sections of MPTP-intoxicated mice were also double-labeled for iNOS and glial cell markers (GFAP for astrocytes and CD11b for microglia). Results represent three independent experiments. B) Cells positive for iNOS were counted in four nigral sections (two images per slide) from each of four mice. ap<0.0001 vs saline-control; bp<0.0001 vs MPTP. The mRNA expression of TNF-α, iNOS and IL-1β was analyzed by semi-quantitative RT-PCR (C) and quantitative real-time PCR (D). Data are means
SEM of five mice per group. ap<0.0001 vs saline group; bp<0.0001 vs the MPTP group.
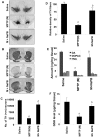
Figure 8. NaPB protects dopaminergic neurons in MPTP-intoxicated mice.
Mice receiving NaPB (200 mg/kg body wt/day) from 3 h after the last injection of MPTP were sacrificed 7 d after the last injection of MPTP followed by TH immunostaining of SNpc (A) and striatum (B), counting of TH-positive neurons in SNpc (C), quantification of TH-positive fibers in striatum (D), assay of neurotransmitters in striatum (E), and quantification of GSH in nigra (F). Data are means
SEM of eight mice per group. ap<0.0001 vs saline group; bp<0.0001 vs the MPTP group.
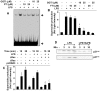
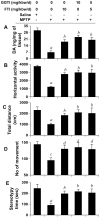
Figure 9. FTI and GGTI protect DA and improve locomotor activities in MPTP-intoxicated mice.
Mice receiving FTI, GGTI or the combination of the two via daily intraperitoneal injection from 3 h after the last injection of MPTP were tested for motor functions (B, horizontal activity; C, total distance; D, number of movement; E, stereotypy) 7 d after the last injection of MPTP followed by measuring DA in striatum (A). Data are means
SEM of eight mice per group. ap<0.001 vs saline; bp<0.001 vs MPTP; cp<0.05 vs saline; dp<0.05 vs MPTP.
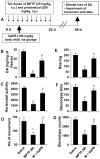
Figure 10. NaPB protects striatal dopamine and improves motor functions in a chronic MPTP mouse model of PD.
A) Six to eight week old male C57BL/6 mice received 10 injections of MPTP (s.c.; 25 mg/kg body weight) together with 10 injections of probenecid (i.p.; 250 mg/kg body weight) for 5 weeks. Control group of mice received only saline. One group of mice were treated with NaPB (100 mg/kg body weight/d) via gavage from the 3rd injection of MPTP/probenecid and continued for 1 week thereafter. Mice were tested for different motor tasks [horizontal activity (C), number of movements (D), rearing (E), and stereotypy (F)] one week after the last injection of MPTP followed by measuring the level of DA in striatum (B). Data are means
SEM of eight mice per group. ap<0.001 vs saline; bp<0.001 vs MPTP; cp<0.05 vs saline; dp<0.05 vs MPTP.
Similar articles
- Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson's disease.
Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K. Ghosh A, et al. J Neurosci. 2009 Oct 28;29(43):13543-56. doi: 10.1523/JNEUROSCI.4144-09.2009. J Neurosci. 2009. PMID: 19864567 Free PMC article. - Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson's disease.
Ghosh A, Kanthasamy A, Joseph J, Anantharam V, Srivastava P, Dranka BP, Kalyanaraman B, Kanthasamy AG. Ghosh A, et al. J Neuroinflammation. 2012 Oct 23;9:241. doi: 10.1186/1742-2094-9-241. J Neuroinflammation. 2012. PMID: 23092448 Free PMC article. - EriB targeted inhibition of microglia activity attenuates MPP+ induced DA neuron injury through the NF-κB signaling pathway.
Dou F, Chu X, Zhang B, Liang L, Lu G, Ding J, Chen S. Dou F, et al. Mol Brain. 2018 Dec 18;11(1):75. doi: 10.1186/s13041-018-0418-z. Mol Brain. 2018. PMID: 30563578 Free PMC article. - Manganese-Enhanced Magnetic Resonance Imaging for Detection of Vasoactive Intestinal Peptide Receptor 2 Agonist Therapy in a Model of Parkinson's Disease.
Olson KE, Bade AN, Schutt CR, Dong J, Shandler SJ, Boska MD, Mosley RL, Gendelman HE, Liu Y. Olson KE, et al. Neurotherapeutics. 2016 Jul;13(3):635-46. doi: 10.1007/s13311-016-0449-z. Neurotherapeutics. 2016. PMID: 27329163 Free PMC article. Review. - Regulation of inducible nitric oxide synthase gene in glial cells.
Saha RN, Pahan K. Saha RN, et al. Antioxid Redox Signal. 2006 May-Jun;8(5-6):929-47. doi: 10.1089/ars.2006.8.929. Antioxid Redox Signal. 2006. PMID: 16771683 Free PMC article. Review.
Cited by
- Nebulization of low-dose aspirin ameliorates Huntington's pathology in N171-82Q transgenic mice.
Mondal S, Prieto S, Rangasamy SB, Dutta D, Pahan K. Mondal S, et al. NeuroImmune Pharm Ther. 2024 Feb 9;3(1):47-59. doi: 10.1515/nipt-2023-0026. eCollection 2024 Mar. NeuroImmune Pharm Ther. 2024. PMID: 38532785 Free PMC article. - Increase in Mitochondrial Biogenesis in Neuronal Cells by RNS60, a Physically-Modified Saline, via Phosphatidylinositol 3-Kinase-Mediated Upregulation of PGC1α.
Chandra G, Kundu M, Rangasamy SB, Dasarathy S, Ghosh S, Watson R, Pahan K. Chandra G, et al. J Neuroimmune Pharmacol. 2018 Jun;13(2):143-162. doi: 10.1007/s11481-017-9771-4. Epub 2017 Nov 29. J Neuroimmune Pharmacol. 2018. PMID: 29188424 Free PMC article. - The Promise of Epigenetic Editing for Treating Brain Disorders.
González Molina LA, Dolga AM, Rots MG, Sarno F. González Molina LA, et al. Subcell Biochem. 2025;108:111-190. doi: 10.1007/978-3-031-75980-2_4. Subcell Biochem. 2025. PMID: 39820862 Review. - Neuroprotection by Epigenetic Modulation in a Transgenic Model of Multiple System Atrophy.
Sturm E, Fellner L, Krismer F, Poewe W, Wenning GK, Stefanova N. Sturm E, et al. Neurotherapeutics. 2016 Oct;13(4):871-879. doi: 10.1007/s13311-016-0447-1. Neurotherapeutics. 2016. PMID: 27259295 Free PMC article. - Propolis induces cardiac metabolism changes in 6-hydroxydopamine animal model: A dietary intervention as a potential cardioprotective approach in Parkinson's disease.
Goncalves VC, Silva da Fonsêca V, de Paula Faria D, Izidoro MA, Berretta AA, de Almeida AG, Affonso Fonseca FL, Scorza FA, Scorza CA. Goncalves VC, et al. Front Pharmacol. 2022 Oct 13;13:1013703. doi: 10.3389/fphar.2022.1013703. eCollection 2022. Front Pharmacol. 2022. PMID: 36313332 Free PMC article.
References
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. - PubMed
- Gao HM, Liu B, Zhang W, Hong JS. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci. 2003;24:395–401. - PubMed
- Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28:502–508. - PubMed
- Zhang W, Wang T, Qin L, Gao HM, Wilson B, et al. Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. Faseb J. 2004;18:589–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 NS071479/NS/NINDS NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- NS71479/NS/NINDS NIH HHS/United States
- NS64564/NS/NINDS NIH HHS/United States
- NS48923/NS/NINDS NIH HHS/United States
- R21 NS064564/NS/NINDS NIH HHS/United States
- R21 NS048923/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous