Phosphoinositide isoforms determine compartment-specific ion channel activity - PubMed (original) (raw)
Phosphoinositide isoforms determine compartment-specific ion channel activity
Xiaoli Zhang et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Phosphoinositides serve as address labels for recruiting peripheral cytoplasmic proteins to specific subcellular compartments, and as endogenous factors for modulating the activity of integral membrane proteins. Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P(2)) is a plasma-membrane (PM)-specific phosphoinositide and a positive cofactor required for the activity of most PM channels and transporters. This requirement for phosphoinositide cofactors has been proposed to prevent PM channel/transporter activity during passage through the biosynthetic/secretory and endocytic pathways. To determine whether intracellularly localized channels are similarly "inactivated" at the PM, we studied PIP(2) modulation of intracellular TRPML1 channels. TRPML1 channels are primarily localized in lysosomes, but can also be detected temporarily in the PM upon lysosomal exocytosis. By directly patch-clamping isolated lysosomes, we previously found that lysosomal, but not PM-localized, TRPML1 is active with PI(3,5)P(2), a lysosome-specific PIP(2), as the underlying positive cofactor. Here we found that "silent" PM-localized TRPML1 could be activated by depleting PI(4,5)P(2) levels and/or by adding PI(3,5)P(2) to inside-out membrane patches. Unlike PM channels, surface-expressed TRPML1 underwent a unique and characteristic run-up upon patch excision, and was potently inhibited by a low micromolar concentration of PI(4,5)P(2). Conversely, depletion of PI(4,5)P(2) by either depolarization-induced activation or chemically induced translocation of 5'-phosphatase potentiated whole-cell TRPML1 currents. PI(3,5)P(2) activation and PI(4,5)P(2) inhibition of TRPML1 were mediated by distinct basic amino acid residues in a common PIP(2)-interacting domain. Thus, PI(4,5)P(2) may serve as a negative cofactor for intracellular channels such as TRPML1. Based on these results, we propose that phosphoinositide regulation sets compartment-specific activity codes for membrane channels and transporters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
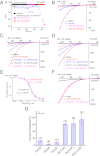
PI(3,5)P2 activates and PI(4,5)P2 inhibits the activity of the PM-localized TRPML1 channel. I/O giant macropatches were excised from the PM of TRPML1-transfected HEK293T cells. The pipette solution contained low pH (4.6) external (modified Tyrode’s) solution, and the bath solution was a K+-based internal/cytoplasmic solution (140 mM K-gluconate; 0 mM ATP). Single channel currents were recorded in the excised patch membranes (HP = –120 mV). C, channel closed state; O, channel open state. (A) Representative trace shows sporadic single-channel openings of TRPML1 (basal NPO = 0.004). Bath application of 300 nM diC8-PI(3,5)P2 to an I/O patch increased NPO to 1.071; coapplication of PI(3,5)P2 and 100 μM SF-51 further enhanced the channel activity (NPO = 2.971); the stimulatory effects were reversible upon washout of the agonists. (B) Single-channel chord conductance of TRPML1 was 45 ± 2 pS (from –140 to –40 mV; n = 6). Single channel TRPML1 currents (_i_ML1) were activated by PI(3,5)P2 in I/O patches at different voltages. (C) _i_ML1 gradually ran up (NPO = 0.001 at 30 s, and 0.379 at 90 s after excision) upon membrane patch excision into the bath solution (0 mM ATP). No significant _i_ML1 openings were detected under the cell-attached configuration. Dashed line indicates the cell-attached configuration; solid line indicates the excised I/O patch configuration. (D) Post–run-up steady-state _i_ML1 was strongly inhibited by 1 μM PI(4,5)P2 (NPO from 0.154 to 0.003). (E) Opposite effects of PI(3,5)P2 and PI(4,5)P2 on post–run-up _i_ML1. Data are presented as mean ± SEM; numbers (n) are noted in parentheses.
Fig. 2.
Agonist-induced TRPML1 channel activity at the PM is suppressed by PI(4,5)P2. (A) _I_ML1-4A gradually underwent run-up in an I/O patch, and were inhibited by PI(4,5)P2 and activated by PI(3,5)P2. _I_ML1-4A were recorded from I/O patches with repeated voltage ramps (from –140 to +140 mV; 400 ms; 4-s interval between ramps). Only a portion of the voltage is shown. Current amplitudes measured at –140 mV were used to plot the time dependence of the PIP2 effects. The post–run-up _I_ML1-4A was strongly inhibited by 300 nM PI(4,5)P2 but robustly reactivated by 100 nM PI(3,5)P2. (B) Representative traces of _I_ML1-4A at the basal level (pre–run-up), post–run-up, and in the presence of PI(4,5)P2 or PI(3,5)P2, as indicated by colored circles in A. (C) Dose-dependent inhibition of PI(3,5)P2-activated _I_ML1-4A by PI(4,5)P2 in an I/O patch from ML1-4A-expressing HEK293 stable cell lines. (D) Dose-dependent inhibition of SF-51–activated _I_ML1-4A by PI(4,5)P2. (E) PI(4,5)P2 inhibited both PI(3,5)P2-activated and SF-51-activated _I_ML1-4A (IC50 = 0.2 μM and 0.18 μM, respectively). (F) PI(3,5)P2-activated _I_ML1-4A was insensitive to PI(4)P (300 nM). (G) Effects of various phosphoinositides on PI(3,5)P2-activated _I_ML1-4A. In all experiments, phosphoinositides (300 nM) were coapplied with PI(3,5)P2 (100 nM). Data are presented as mean ± SEM; numbers (n) are noted in parentheses.
Fig. 3.
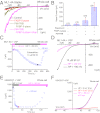
Depletion of PI(4,5)P2 levels increases whole-cell TRPML1 currents. (A) Overexpression of cytosolic FKBP-5′-phosphatase along with rapamycin-induced translocation to the PM enhanced basal whole-cell _I_ML1-4A. _I_ML1-4A was measured in the whole-cell configuration with repeated voltage ramps (from –140 to +140 mV; 400 ms; 4-s interval between ramps; HP = 0 mV). Only a portion of the voltage is shown. The pipette solution contained Cs+-based internal solution (145 mM Cs-Mes), and the bath was standard external (Tyrode’s) solution. HEK293 cells stably expressing ML1–4A were transfected with RFP-FKBP-5-phosphatase alone or together with CFP-PM-FRB, which contains a signal for the PM localization. The dimerization of FRB and FKBP induced by rapamycin (0.5 μM for 10 min) led to translocation of the 5-phosphatase from the cytosol to the PM. (B) Summary effects of overexpression and PM translocation of 5-phosphatase on basal _I_ML1-4A. Rapamycin significantly enhanced basal _I_ML1-4A. (C) Depolarization-activated Ci-VSP 5-phosphatase increased SF-51–activated _I_ML1-4A in Ci-VSP–transfected ML1–4A stable cells. _I_ML1-4A was elicited by repeated voltage ramps (from –140 mV to +100 mV; 100-ms duration; 10-s interval between ramps; HP = –60 mV or +80 mV). Current amplitudes measured at −140 mV were used to plot the time-dependence of the depolarization-induced effect. (D) Representative traces of SF-51-activated _I_ML1-4A before (HP = −60 mV, red) and 8 min after (HP = +80 mV, black) Ci-VSP activation at two time points, as shown in C. (E) Activation of Ci-VSP (HP = 0 mV) led to rapid suppression of endogenous TRPM7-like currents in Ci-VSP–transfected HEK293T cells. TRPM7-like currents were induced by omission of Mg2+ in both bath and internal/pipette solutions (HP = -60 mV for >3 min after break-in), and then elicited by repeated voltage ramps (from –100 mV to +120 mV; 100-ms duration; 1- or 10-s interval between ramps; HP = 0 mV or –60 mV). (F) Representative I-V traces of TRPM7-like currents before (HP = 0 mV, 0 s) after Ci-VSP activation (HP = 0 mV, 25 s), and after Ci-VSP deactivation (HP = –60 mV, 25 s) at three time points, as shown in E. Data are presented as mean ± SEM; numbers (n) are noted in parentheses. Statistical comparisons were performed using analysis of variance. *P < 0.05.
Fig. 4.
Structural determinants of PI(4,5)P2 inhibition of TRPML1. (A) In an I/O patch from ML1–4A–expressing HEK293 cells, _I_ML1-4A activated by SF-51 (25 μM; bath application) was significantly inhibited by PI(4,5)P2 (0.5 μM). PI(3,5)P2 (1 μM) activated _I_ML1-4A in the same patch. (B) SF-51–activated _I_ML1-4A-7Q was insensitive to PI(4,5)P2 (0.5 μM), and _I_ML1-4A-7Q was not activated by PI(3,5)P2 (1 μM) in the same patch. (C) Bar graph depicting the insensitivity of ML1-4A-7Q to PI(4,5)P2. (D and E) 7Q mutations abolished the effect of Ci-VSP activation (HP = +80 mV, 8 min) of ML1–4A. (F) 7Q mutations and triple mutations (R42A/R43A/R44A) abolished the stimulatory effect of Ci-VSP on ML1–4A. (G) ML1–4A/42–44A was only slightly inhibited by PI(4,5)P2, but robustly activated by PI(3,5)P2. (H) Bar graph depicting the sensitivity of ML1–4A/42–44A and ML1–4A/61–62A to PI(4,5)P2 inhibition (ML1–4A data were replotted from C for comparison). (I) ML1–4A/42–44A, but not ML1–4A/61–62A, remained sensitive to PI(3,5)P2 activation. Data are presented as mean ± SEM; numbers (n) are noted in parentheses. Statistical comparisons were performed using analysis of variance. *P < 0.05.
Similar articles
- Drosophila TRPML forms PI(3,5)P2-activated cation channels in both endolysosomes and plasma membrane.
Feng X, Huang Y, Lu Y, Xiong J, Wong CO, Yang P, Xia J, Chen D, Du G, Venkatachalam K, Xia X, Zhu MX. Feng X, et al. J Biol Chem. 2014 Feb 14;289(7):4262-72. doi: 10.1074/jbc.M113.506501. Epub 2013 Dec 27. J Biol Chem. 2014. PMID: 24375408 Free PMC article. - PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome.
Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. Dong XP, et al. Nat Commun. 2010 Jul 13;1(4):38. doi: 10.1038/ncomms1037. Nat Commun. 2010. PMID: 20802798 Free PMC article. - Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides.
Borbiro I, Badheka D, Rohacs T. Borbiro I, et al. Sci Signal. 2015 Feb 10;8(363):ra15. doi: 10.1126/scisignal.2005667. Sci Signal. 2015. PMID: 25670203 Free PMC article. - TRPML2 and mucolipin evolution.
García-Añoveros J, Wiwatpanit T. García-Añoveros J, et al. Handb Exp Pharmacol. 2014;222:647-58. doi: 10.1007/978-3-642-54215-2_25. Handb Exp Pharmacol. 2014. PMID: 24756724 Review. - Molecular characteristics of phosphoinositide binding.
Rosenhouse-Dantsker A, Logothetis DE. Rosenhouse-Dantsker A, et al. Pflugers Arch. 2007 Oct;455(1):45-53. doi: 10.1007/s00424-007-0291-6. Epub 2007 Jun 23. Pflugers Arch. 2007. PMID: 17588168 Review.
Cited by
- Structural biology of cation channels important for lysosomal calcium release.
Gan N, Jiang Y. Gan N, et al. Cell Calcium. 2022 Jan;101:102519. doi: 10.1016/j.ceca.2021.102519. Epub 2021 Dec 14. Cell Calcium. 2022. PMID: 34952412 Free PMC article. Review. - Cryo-EM structures of the human endolysosomal TRPML3 channel in three distinct states.
Zhou X, Li M, Su D, Jia Q, Li H, Li X, Yang J. Zhou X, et al. Nat Struct Mol Biol. 2017 Dec;24(12):1146-1154. doi: 10.1038/nsmb.3502. Epub 2017 Nov 6. Nat Struct Mol Biol. 2017. PMID: 29106414 Free PMC article. - Bidirectional Allosteric Coupling between PIP2 Binding and the Pore of the Oncochannel TRPV6.
Humer C, Radiskovic T, Horváti K, Lindinger S, Groschner K, Romanin C, Höglinger C. Humer C, et al. Int J Mol Sci. 2024 Jan 3;25(1):618. doi: 10.3390/ijms25010618. Int J Mol Sci. 2024. PMID: 38203789 Free PMC article. - Regulation of V-ATPase Activity and Organelle pH by Phosphatidylinositol Phosphate Lipids.
Banerjee S, Kane PM. Banerjee S, et al. Front Cell Dev Biol. 2020 Jun 23;8:510. doi: 10.3389/fcell.2020.00510. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32656214 Free PMC article. Review. - A New Perspective of Lysosomal Cation Channel-Dependent Homeostasis in Alzheimer's Disease.
Ezeani M, Omabe M. Ezeani M, et al. Mol Neurobiol. 2016 Apr;53(3):1672-1678. doi: 10.1007/s12035-015-9108-3. Epub 2015 Feb 18. Mol Neurobiol. 2016. PMID: 25691454 Review.
References
- Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. - PubMed
- Montell C. An exciting release on TRPM7. Neuron. 2006;52:395–397. - PubMed
- Dellis O, et al. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous