Development of a multi-step leukemogenesis model of MLL-rearranged leukemia using humanized mice - PubMed (original) (raw)
doi: 10.1371/journal.pone.0037892. Epub 2012 Jun 20.
Makiko Suzuki, Yohei Watanabe, Takeshi Takahashi, Yoko Aoki, Toru Uchiyama, Satoru Kumaki, Yoji Sasahara, Masayoshi Minegishi, Shigeo Kure, Shigeru Tsuchiya, Kazuo Sugamura, Naoto Ishii
Affiliations
- PMID: 22745659
- PMCID: PMC3380045
- DOI: 10.1371/journal.pone.0037892
Development of a multi-step leukemogenesis model of MLL-rearranged leukemia using humanized mice
Kunihiko Moriya et al. PLoS One. 2012.
Abstract
Mixed-lineage-leukemia (MLL) fusion oncogenes are intimately involved in acute leukemia and secondary therapy-related acute leukemia. To understand MLL-rearranged leukemia, several murine models for this disease have been established. However, the mouse leukemia derived from mouse hematopoietic stem cells (HSCs) may not be fully comparable with human leukemia. Here we developed a humanized mouse model for human leukemia by transplanting human cord blood-derived HSCs transduced with an MLL-AF10 oncogene into a supra-immunodeficient mouse strain, NOD/Shi-scid, IL-2Rγ(-/-) (NOG) mice. Injection of the MLL-AF10-transduced HSCs into the liver of NOG mice enhanced multilineage hematopoiesis, but did not induce leukemia. Because active mutations in ras genes are often found in MLL-related leukemia, we next transduced the gene for a constitutively active form of K-ras along with the MLL-AF10 oncogene. Eight weeks after transplantation, all the recipient mice had developed acute monoblastic leukemia (the M5 phenotype in French-American-British classification). We thus successfully established a human MLL-rearranged leukemia that was derived in vivo from human HSCs. In addition, since the enforced expression of the mutant K-ras alone was insufficient to induce leukemia, the present model may also be a useful experimental platform for the multi-step leukemogenesis model of human leukemia.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
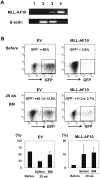
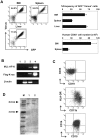
Figure 1. Enforced expression of MLL-AF10 augmented multilineage hematopoiesis, but was insufficient to induce leukemogenesis in vivo.
(A) Representative RT-PCR results confirming the long-term expression of the MLL-AF10 transcript in the BM cells of mice 25 weeks after transplantation (lane 1; water, lane 2; cells from a mouse in the EV-transfused group, lane 3; cells from a mouse in the MLL-AF10-transfused group, and lane 4; positive control (MLL-AF10 plasmid)). (B) Flowcytometric analysis of the frequency of GFP+ cells. The indicated vector (EV, left or MLL-AF10, right)-transduced human CD34+ cells, whose in vitro GFP expression is shown in the upper panels (Before) of the flowcytometric analysis, were transplanted into NOG mice. Twenty-five weeks later, the GFP-expressing cells gated on human CD45+ hematopoietic cells in the BM was measured (lower panels of the FACS profiles). The data shown are representative of 3 independent experiments. The graphs show the frequency of GFP+ cells in human CD34+ cells just before transplantation (Before) and the mean ± SD of the frequency of GFP+ cells in the BM and spleen of mice receiving transplants of EV-transduced HSCs (n = 8) or of MLL-AF10-transduced HSCs (n = 6) 25 weeks after transplantation, in one representative experiment of three. Similar results were obtained in the 3 independent experiments.
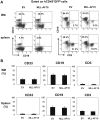
Figure 2. Flowcytometric analysis confirming multilineage engraftment.
(A) Representative flowcytometric results of EV- or MLL-AF10-transduced human hematopoietic cells. The human CD45+ GFP+ cells were analyzed for their lineage distributions to B cells (CD19+), T cells (CD3+), and myeloid cells (CD33+). (B) Multilineage differentiation of MLL-AF10-transduced cells. The data shows cells gated on the CD45+GFP+ cell population. The graph represents the mean ± SD of the frequencies of CD33+ myeloid cells, CD19+ B cells, and CD3+ T cells in the BM (upper) and spleens (lower) of mice engrafted with EV-transduced (n = 8) or MLL-AF10-transduced (n = 6) CD34+ HSCs. No difference in the graft composition between the EV- and MLL-AF10-expressing CD34+ HSCs was found. Similar results were obtained in 3 independent experiments.
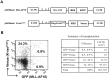
Figure 3. Co-transduction of activated K-ras and MLL-AF10 into CD34+HSCs.
(A) Schematic structure of the MLL-AF10-GFP and Flag-K-rasG12V-Venus vectors. (B) Infectious efficiency of the MLL-AF10-GFP and Flag-K-rasG12V-Venus co-transfection. The data and the summary shown in the flowcytometric analysis is representative of the transduced CD34+ HSCs in 2 experiments.
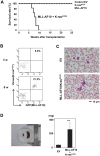
Figure 4. Cooperation of MLL-AF10 with activated K-ras induced acute monoblastic leukemia.
(A) Kaplan-Meier survival analysis of mice receiving transplants of human HSCs transfected with EV (n = 8), K-ras G12V (n = 12), MLL-AF10 (n = 6), or MLL-AF10 plus K-rasG12V (n = 6) vectors. (B) GFP and Venus expression in peripheral blood cells at the indicated weeks after transplantation with human HSCs co-transfected with the MLL-AF10 and K-rasG12V genes. (C) May-Giemsa staining of the peripheral blood of mice engrafted with human HSCs co-transfected with the MLL-AF10 and K-rasG12V genes. Morphologic leukemia cells were found in the peripheral blood of these mice 50 days after transplantation. (D) Splenomegaly in the MLL-AF10/K-rasG12V mice. Spleens from mice engrafted with EV-transduced HSCs (left) and MLL-AF10/K-rasG12V co-transduced HSCs (right) are shown. The graph shows the mean ± SD of the spleen weights from mice receiving transplants of EV-transduced HSCs (n = 6) or of MLL-AF10/K-rasG12V co-transduced HSCs (n = 6). ** represents p<0.01.
Figure 5. Immunophenotype and clonality of the MLL-AF10/K-ras-induced leukemia.
(A) Frequencies of GFP+/Venus+ cells or human CD45+ cells in the BM, spleen, and liver at 8 weeks after transplantation with human HSCs co-transfected with the MLL-AF10 and K-rasG12V genes were examined by flowcytometric analysis. The flowcytometry data shown are representative of 6 to 8 mice per group in one representative experiment of two (left). The average of %frequencies of the GFP+ and Venus+ cells in whole cells in the indicated organs is shown with the standard deviation (right, upper; n = 6). The absolute cell number of human CD45+ cells in the indicated organs is shown with the standard deviation (right, lower; n = 6). (B) Representative RT-PCR results confirming the stable, long-term expression of the MLL-AF10 and Flag-K-rasG12V transcripts in human hematopoietic cells in the BM of mice 8 weeks after transplantation. (C) Lineage distribution of the GFP+ and Venus+ cells in the BM of a mouse engrafted with HSCs expressing MLL-AF10 and activated K-ras. (D) Southern blot analysis of DNA prepared from the human blood cells in the spleen of mice receiving transplants of MLL-AF10/K-rasG12V co-transduced HSCs. Independent leukemia samples derived from two mice (lane 1; mouse 1 and lane 2; mouse 2) were examined. DNA was digested with Bgl II and probed with an EGFP probe. M: marker.
Figure 6. Pathological phenotypes of the leukemia.
(A) Hematoxylin and eosin staining showing the infiltration of leukemic cells in the indicated organs of mice engrafted with HSCs expressing the MLL-AF10 and K-rasG12V genes compared to control mice. (B) Immunostaining by a human CD45 mAb in the BM, spleen, and liver in mice engrafted with HSCs expressing the MLL-AF10 and K-rasG12V genes.
Similar articles
- Plzf drives MLL-fusion-mediated leukemogenesis specifically in long-term hematopoietic stem cells.
Ono R, Masuya M, Nakajima H, Enomoto Y, Miyata E, Nakamura A, Ishii S, Suzuki K, Shibata-Minoshima F, Katayama N, Kitamura T, Nosaka T. Ono R, et al. Blood. 2013 Aug 15;122(7):1271-83. doi: 10.1182/blood-2012-09-456665. Epub 2013 Jul 9. Blood. 2013. PMID: 23838347 - MLL-AF9 and FLT3 cooperation in acute myelogenous leukemia: development of a model for rapid therapeutic assessment.
Stubbs MC, Kim YM, Krivtsov AV, Wright RD, Feng Z, Agarwal J, Kung AL, Armstrong SA. Stubbs MC, et al. Leukemia. 2008 Jan;22(1):66-77. doi: 10.1038/sj.leu.2404951. Epub 2007 Sep 13. Leukemia. 2008. PMID: 17851551 Free PMC article. - Tet1 is not required for myeloid leukemogenesis by MLL-ENL in novel mouse models.
Ono R, Masuya M, Inoue N, Shinmei M, Ishii S, Maegawa Y, Maharjan BD, Katayama N, Nosaka T. Ono R, et al. PLoS One. 2021 Mar 11;16(3):e0248425. doi: 10.1371/journal.pone.0248425. eCollection 2021. PLoS One. 2021. PMID: 33705482 Free PMC article. - Learning from mouse models of MLL fusion gene-driven acute leukemia.
Schwaller J. Schwaller J. Biochim Biophys Acta Gene Regul Mech. 2020 Aug;1863(8):194550. doi: 10.1016/j.bbagrm.2020.194550. Epub 2020 Apr 19. Biochim Biophys Acta Gene Regul Mech. 2020. PMID: 32320749 Review. - Transcriptional activation by MLL fusion proteins in leukemogenesis.
Yokoyama A. Yokoyama A. Exp Hematol. 2017 Feb;46:21-30. doi: 10.1016/j.exphem.2016.10.014. Epub 2016 Nov 16. Exp Hematol. 2017. PMID: 27865805 Review.
Cited by
- Use of Genome Engineering to Create Patient Specific MLL Translocations in Primary Human Hematopoietic Stem and Progenitor Cells.
Breese EH, Buechele C, Dawson C, Cleary ML, Porteus MH. Breese EH, et al. PLoS One. 2015 Sep 9;10(9):e0136644. doi: 10.1371/journal.pone.0136644. eCollection 2015. PLoS One. 2015. PMID: 26351841 Free PMC article. - Genetic hierarchy and temporal variegation in the clonal history of acute myeloid leukaemia.
Hirsch P, Zhang Y, Tang R, Joulin V, Boutroux H, Pronier E, Moatti H, Flandrin P, Marzac C, Bories D, Fava F, Mokrani H, Betems A, Lorre F, Favier R, Féger F, Mohty M, Douay L, Legrand O, Bilhou-Nabera C, Louache F, Delhommeau F. Hirsch P, et al. Nat Commun. 2016 Aug 18;7:12475. doi: 10.1038/ncomms12475. Nat Commun. 2016. PMID: 27534895 Free PMC article. - De novo activating mutations drive clonal evolution and enhance clonal fitness in KMT2A-rearranged leukemia.
Hyrenius-Wittsten A, Pilheden M, Sturesson H, Hansson J, Walsh MP, Song G, Kazi JU, Liu J, Ramakrishan R, Garcia-Ruiz C, Nance S, Gupta P, Zhang J, Rönnstrand L, Hultquist A, Downing JR, Lindkvist-Petersson K, Paulsson K, Järås M, Gruber TA, Ma J, Hagström-Andersson AK. Hyrenius-Wittsten A, et al. Nat Commun. 2018 May 2;9(1):1770. doi: 10.1038/s41467-018-04180-1. Nat Commun. 2018. PMID: 29720585 Free PMC article. - Myeloid malignancies with translocation t(4;12)(q11-13;p13): molecular landscape, clonal hierarchy and clinical outcomes.
Parinet V, Chapiro E, Bidet A, Gaillard B, Maarek O, Simon L, Lefebvre C, Defasque S, Mozziconacci MJ, Quinquenel A, Decamp M, Lifermann F, Ali-Ammar N, Maillon A, Baron M, Martin M, Struski S, Penther D, Micol JB, Auger N, Bilhou-Nabera C, Martignoles JA, Tondeur S, Nguyen-Khac F, Hirsch P, Roos-Weil D; on behalf the FILO (French Innovative Leukemia Organization), GFCH (Groupe Francophone de Cytogénétique Hématologique) groups. Parinet V, et al. J Cell Mol Med. 2021 Oct;25(20):9557-9566. doi: 10.1111/jcmm.16895. Epub 2021 Sep 7. J Cell Mol Med. 2021. PMID: 34492730 Free PMC article. - The molecular basis of myeloid malignancies.
Kitamura T, Inoue D, Okochi-Watanabe N, Kato N, Komeno Y, Lu Y, Enomoto Y, Doki N, Uchida T, Kagiyama Y, Togami K, Kawabata KC, Nagase R, Horikawa S, Hayashi Y, Saika M, Fukuyama T, Izawa K, Oki T, Nakahara F, Kitaura J. Kitamura T, et al. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(10):389-404. doi: 10.2183/pjab.90.389. Proc Jpn Acad Ser B Phys Biol Sci. 2014. PMID: 25504228 Free PMC article. Review.
References
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nature Reviews Cancer. 2007;7:823– 833. - PubMed
- Meyer C, Kowarz E, Hofmann J, Renneville A, Zuna J, et al. New insights to the MLL recombinome of acute leukemias. Leukemia. 2009;23:1490– 1499. - PubMed
- Balgobind BV, Zwaan CM, Pieters R, Van den Heuvel-Eibrink MM. The heterogeneity of pediatric MLL-rearranged acute myeloid leukemia. Leukemia. 2011;25:1239– 1248. - PubMed
- Tomizawa D, Koh K, Sato T, Kinukawa N, Morimoto A, et al. Outcome of risk-based therapy for infant acute lymphoblastic leukemia with or without an MLL gene rearrangement, with emphasis on late effects: a final report of two consecutive studies, MLL96 and MLL98, of the Japan Infant Leukemia Study Group. Leukemia. 2007;21:2258– 2263. - PubMed
- Johnson JJ, Chen W, Hudson W, Yao Q, Taylor M, et al. Prenatal and postnatal myeloid cells demonstrate stepwise progression in the pathogenesis of MLL fusion gene leukemia. Blood. 2003;101:3229– 3235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous