Nod1, but not the ASC inflammasome, contributes to induction of IL-1β secretion in human trophoblasts after sensing of Chlamydia trachomatis - PubMed (original) (raw)
Nod1, but not the ASC inflammasome, contributes to induction of IL-1β secretion in human trophoblasts after sensing of Chlamydia trachomatis
P B Kavathas et al. Mucosal Immunol. 2013 Mar.
Abstract
Chlamydia trachomatis (Ct) is an obligate intracellular bacterial pathogen. Previously, we showed that infection of human trophoblast cells by Ct triggers the secretion of the pro-inflammatory cytokine, interleukin (IL)-1β. The aim of this study was to understand the innate immune pathways involved in trophoblast production of IL-1β after Ct infection. The approach we took was to inhibit the expression or function of the key Toll-like receptors (TLRs), Nod-like receptors, and inflammasome components that have been associated with chlamydia infection. In this study, we report that Ct-induced trophoblast IL-1β secretion is associated with the transcription of IL-1β mRNA, the translation and processing of pro-IL-1β, and the activation of caspase-1. In addition, we demonstrate that Ct-induced IL-1β production and secretion by the trophoblast is independent of TLR2, TLR4, MyD88, and the Nalp3/ASC inflammasome. Instead we report, for the first time, the importance of Nod1 for mediating trophoblast IL-1β secretion in response to a Ct infection.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
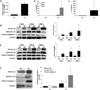
Figure 1. Chlamydia infection of human trophoblast cell lines leads to induction of IL-1β expression and IL-1β processing
Trophoblast cells were either non-infected (NI) or infected with Chlamydia (Ct), after which supernatants were collected and either RNA or protein extracted from cells. (a) Ct infection of HTR8 cells significantly induced IL-1β mRNA expression after 24hrs as determined by qRT-PCR; and (b) significantly induced IL-1β secretion after 48hrs as determined by ELISA. (c) Ct infection of Sw.71 cells significantly induced IL-1β secretion after 36hrs (n=3; *p<0.05 relative to the NI control). Cell lysates from (d) HTR8 and (e) Sw.71 cells after either NI or infection with Ct were evaluated for pro-IL-1β (31kDa), active IL-1β (17kDa) and active caspase-1 (20kDa) expression by Western blot (representative blots are shown). Barcharts show quantification of protein expression as determined by densitometry and normalized to β-actin (n=3; *p<0.05 relative to the NI control). (f) Cell supernatants from Sw.71 cells either NI or infected with Ct for 36hrs were evaluated for pro- and active IL-1β and active caspase-1 expression by Western blot. Barcharts show quantification of protein expression as determined by densitometry and normalized to β-actin (n=4; *p<0.05 relative to the NI control).
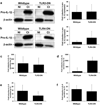
Figure 2. _Chlamydia_-induced trophoblast pro-IL-1β expression and secretion of IL-1β and IL-8 is independent of TLR2 and TLR4
Wildtype trophoblast (3A and HTR8) and trophoblast cells transfected to express either a TLR2-dominant negative (DN) (in 3A cells) or a TLR4-DN (in HTR8 cells) were either NI or infected with Ct for 48hrs. (a & b) Cell lysates were evaluated for pro-IL-1β expression by Western blot (representative blots are shown). Barcharts show quantification of pro-IL-1β levels, as determined by densitometry and normalized to β-actin. Culture supernatants were analyzed for (c & d) IL-1β and (e & f) IL-8 levels by ELISA. Data are pooled from three independent experiments and no significance was observed between the response of wildtype and TLR2-DN or TLR4-DN cells to Ct infection.
Figure 3. _Chlamydia_-induced trophoblast IL-8 secretion, but not IL-1β expression or secretion is dependent on MyD88
Wildtype HTR8 trophoblast and HTR8 cells transfected to express a MyD88-DN were either NI or infected with Ct for 48hrs. (a) RNA was analyzed for IL-1β mRNA levels by qRT-PCR; (b) cell lysates were analyzed for pro-IL-1β expression levels by Western blot (representative blots are shown) and densitometry; and culture supernatants were measured for (c) IL-1β and (d) IL-8 by ELISA. Data are pooled from three independent experiments; *p<0.05 relative to the wildtype cells.
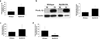
Figure 4. _Chlamydia_-induced trophoblast IL-1β secretion is not dependent on ASC or Nalp3
(a–b) Sw.71 trophoblast cells were transfected to express either shRNA for ASC (sh-ASC) or a control sequence (sh-control) (a) Western blot of lysates from the Sw.71 trophoblast cells expressing either sh-control or sh-ASC for ASC expression. β-actin served as a loading control. (b) 36 hrs after either NI or Ct infection, levels of secreted IL-1β in the supernatants of sh-control and sh-ASC cells were measured by ELISA. n=3; *p<0.05 relative to the NI control. (c–d) Sw.71 trophoblast cells were transfected to express either shRNA for Nalp3 (sh-Nalp3) or a control sequence (sh-control). (c) RNA from the Sw.71 trophoblast cells expressing either sh-control or sh-NAlp3 was analyzed for Nalp3 mRNA expression by qRT-PCR. n=3; *p<0.05 relative to the sh-control. (d) 72hrs after either no treatment (NT) or treatment with MSU (100µg/ml) levels of secreted IL-1β in the supernatants of sh-control and sh-Nalp3 cells were measured by ELISA. n=3; *p<0.05 relative to the NT control unless otherwise indicated. (e) 36 hrs after either NI or Ct infection, levels of secreted IL-1β in the supernatants of sh-control and sh-Nalp3 cells were measured by ELISA. n=3; *p<0.05 relative to the NI control.
Figure 5. C_hlamydia_-induced trophoblast IL-1b processing and secretion is dependent on Nod1
Sw.71 trophoblast cells were transfected to express either shRNA for Nod1 (sh-Nod1) or a control sequence (sh-control) and were either NI or infected with Ct for 36hrs. (a) Expression of Nod1 protein in the sh-control and sh-Nod1 cells was determined by Western blot analysis, and barchart shows densitometry with Nod1 expression normalized to β-actin levels. (b–d) Barcharts show levels of secreted IL-1β, IL-8 and G-CSF in the supernatants of the sh-control and sh-Nod1 cells. (e) Supernatants were evaluated for pro- and active IL-1β and active caspase-1 expression by Western blot. Barcharts show quantification of protein expression in the sh-control and sh-Nod1 cells as determined by densitometry and normalized to β-actin. n=4; *p<0.05; **p<0.001 relative to the NI control unless otherwise indicated.
Similar articles
- Uric acid induces trophoblast IL-1β production via the inflammasome: implications for the pathogenesis of preeclampsia.
Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, Tadesse S, Norwitz ER, Guller S, Abrahams VM. Mulla MJ, et al. Am J Reprod Immunol. 2011 Jun;65(6):542-8. doi: 10.1111/j.1600-0897.2010.00960.x. Am J Reprod Immunol. 2011. PMID: 21352397 Free PMC article. - A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1β production by human first trimester trophoblast.
Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, Abrahams VM. Mulla MJ, et al. PLoS One. 2013 Jun 6;8(6):e65237. doi: 10.1371/journal.pone.0065237. Print 2013. PLoS One. 2013. PMID: 23762324 Free PMC article. - TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection.
Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. Segovia J, et al. PLoS One. 2012;7(1):e29695. doi: 10.1371/journal.pone.0029695. Epub 2012 Jan 25. PLoS One. 2012. PMID: 22295065 Free PMC article. - Murine Borrelia arthritis is highly dependent on ASC and caspase-1, but independent of NLRP3.
Oosting M, Buffen K, Malireddi SR, Sturm P, Verschueren I, Koenders MI, van de Veerdonk FL, van der Meer JW, Netea MG, Kanneganti TD, Joosten LA. Oosting M, et al. Arthritis Res Ther. 2012 Nov 13;14(6):R247. doi: 10.1186/ar4090. Arthritis Res Ther. 2012. PMID: 23148704 Free PMC article.
Cited by
- Immunopathogenesis of Chlamydial Infections.
Murthy AK, Li W, Ramsey KH. Murthy AK, et al. Curr Top Microbiol Immunol. 2018;412:183-215. doi: 10.1007/82_2016_18. Curr Top Microbiol Immunol. 2018. PMID: 27370346 Free PMC article. Review. - Viral single stranded RNA induces a trophoblast pro-inflammatory and antiviral response in a TLR8-dependent and -independent manner.
Potter JA, Garg M, Girard S, Abrahams VM. Potter JA, et al. Biol Reprod. 2015 Jan;92(1):17. doi: 10.1095/biolreprod.114.124032. Epub 2014 Nov 26. Biol Reprod. 2015. PMID: 25429091 Free PMC article. - A Role for the Inflammasome in Spontaneous Labor at Term.
Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, Tarca AL, Abrahams VM, Hassan SS, Yeo L, Gomez-Lopez N. Romero R, et al. Am J Reprod Immunol. 2018 Jun;79(6):e12440. doi: 10.1111/aji.12440. Epub 2016 Mar 8. Am J Reprod Immunol. 2018. PMID: 26952361 Free PMC article. - Chlamydial Plasmid-Dependent Pathogenicity.
Zhong G. Zhong G. Trends Microbiol. 2017 Feb;25(2):141-152. doi: 10.1016/j.tim.2016.09.006. Epub 2016 Oct 3. Trends Microbiol. 2017. PMID: 27712952 Free PMC article. Review. - Clear Victory for Chlamydia: The Subversion of Host Innate Immunity.
Chen H, Wen Y, Li Z. Chen H, et al. Front Microbiol. 2019 Jul 3;10:1412. doi: 10.3389/fmicb.2019.01412. eCollection 2019. Front Microbiol. 2019. PMID: 31333596 Free PMC article. Review.
References
- Mylonas I. Female genital Chlamydia trachomatis infection: where are we heading? Arch Gynecol Obstet. 2012 Feb 19; [Epub ahead of print] - PubMed
- Wiesenfeld HC, Hillier SL, Krohn MA, Amortegui AJ, Heine RP, Landers DV, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100(3):456–463. - PubMed
- Baud D, Regan L, Greub G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr Opin Infect Dis. 2008;21(1):70–76. - PubMed
- Howie SE, Horner PJ, Horne AW. Chlamydia trachomatis infection during pregnancy: known unknowns. Discov Med. 2011;12(62):57–64. - PubMed
- Dong ZW, Li Y, Zhang LY, Liu RM. Detection of Chlamydia trachomatis intrauterine infection using polymerase chain reaction on chorionic villi. Int J Gynaecol Obstet. 1998;61(1):29–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD049446/HD/NICHD NIH HHS/United States
- R01AI049571/AI/NIAID NIH HHS/United States
- R01 AI049571/AI/NIAID NIH HHS/United States
- P01 HD054713/HD/NICHD NIH HHS/United States
- R01HD049446/HD/NICHD NIH HHS/United States
- P01HD054713/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous