A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer - PubMed (original) (raw)
Clinical Trial
. 2012 Aug;134(3):1149-59.
doi: 10.1007/s10549-012-2126-1. Epub 2012 Jul 5.
Ahmad Awada, Philipp Harter, Jean Luc Canon, Kurt Possinger, Marcus Schmidt, Jacques De Grève, Patrick Neven, Luc Dirix, Walter Jonat, Matthias W Beckmann, Jochen Schütte, Peter A Fasching, Nina Gottschalk, Tatiana Besse-Hammer, Frank Fleischer, Sven Wind, Martina Uttenreuther-Fischer, Martine Piccart, Nadia Harbeck
Affiliations
- PMID: 22763464
- PMCID: PMC3409367
- DOI: 10.1007/s10549-012-2126-1
Clinical Trial
A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer
Martin Schuler et al. Breast Cancer Res Treat. 2012 Aug.
Abstract
Afatinib (BIBW 2992) is an ErbB-family blocker that irreversibly inhibits signaling from all relevant ErbB-family dimers. Afatinib has demonstrated preclinical activity in human epidermal growth factor receptor HER2 (ErbB2)-positive and triple-negative xenograft models of breast cancer, and clinical activity in phase I studies. This was a multicenter phase II study enrolling patients with HER2-negative metastatic breast cancer progressing following no more than three lines of chemotherapy. No prior epidermal growth factor receptor-targeted therapy was allowed. Patients received 50-mg afatinib once daily until disease progression. Tumor assessment was performed at every other 28-day treatment course. The primary endpoint was clinical benefit (CB) for ≥4 treatment courses in triple-negative (Cohort A) metastatic breast cancer (TNBC) and objective responses measured by Response Evaluation Criteria in Solid Tumors in patients with HER2-negative, estrogen receptor-positive, and/or progesterone receptor-positive breast cancer (Cohort B). Fifty patients received treatment, including 29 patients in Cohort A and 21 patients in Cohort B. No objective responses were observed in either cohort. Median progression-free survival was 7.4 and 7.7 weeks in Cohorts A and B, respectively. Three patients with TNBC had stable disease for ≥4 treatment courses, one of them for 12 courses (median 26.3 weeks; range 18.9-47.9 weeks). The most frequently observed afatinib-associated adverse events (AEs) were gastrointestinal and skin-related side effects, which were manageable by symptomatic treatment and dose reductions. Afatinib pharmacokinetics were comparable to those observed in previously reported phase I trials. In conclusion, afatinib had limited activity in HER2-negative breast cancer. AEs were generally manageable and mainly affected the skin and the gastrointestinal tract.
Figures
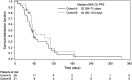
Fig. 1
Kaplan–Meier curves for progression-free survival (treated set)
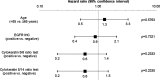
Fig. 2
Hazard ratio (95 % CI) of progression-free survival by baseline characteristics for Cohort A. EGFR epidermal growth factor receptor, IHC immunohistochemistry
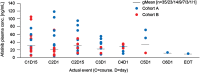
Fig. 3
Predose afatinib plasma concentrations at steady state (50 mg). HER human epidermal growth factor receptor, HR hormone receptor
Similar articles
- A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab.
Lin NU, Winer EP, Wheatley D, Carey LA, Houston S, Mendelson D, Munster P, Frakes L, Kelly S, Garcia AA, Cleator S, Uttenreuther-Fischer M, Jones H, Wind S, Vinisko R, Hickish T. Lin NU, et al. Breast Cancer Res Treat. 2012 Jun;133(3):1057-65. doi: 10.1007/s10549-012-2003-y. Epub 2012 Mar 15. Breast Cancer Res Treat. 2012. PMID: 22418700 Free PMC article. Clinical Trial. - Afatinib alone or afatinib plus vinorelbine versus investigator's choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial.
Cortés J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, Le Rhun E, Espié M, Kim SB, Schneeweiss A, Sohn JH, Nabholtz JM, Kellokumpu-Lehtinen PL, Taguchi J, Piacentini F, Ciruelos E, Bono P, Ould-Kaci M, Roux F, Joensuu H. Cortés J, et al. Lancet Oncol. 2015 Dec;16(16):1700-10. doi: 10.1016/S1470-2045(15)00373-3. Epub 2015 Nov 17. Lancet Oncol. 2015. PMID: 26596672 Clinical Trial. - Afatinib alone and in combination with vinorelbine or paclitaxel, in patients with HER2-positive breast cancer who failed or progressed on prior trastuzumab and/or lapatinib (LUX-Breast 2): an open-label, multicenter, phase II trial.
Hickish T, Mehta A, Liu MC, Huang CS, Arora RS, Chang YC, Yang Y, Vladimirov V, Jain M, Tsang J, Pemberton K, Sadrolhefazi B, Jin X, Tseng LM. Hickish T, et al. Breast Cancer Res Treat. 2022 Apr;192(3):593-602. doi: 10.1007/s10549-021-06449-4. Epub 2022 Feb 9. Breast Cancer Res Treat. 2022. PMID: 35138529 Free PMC article. Clinical Trial. - Afatinib in the treatment of breast cancer.
Hurvitz SA, Shatsky R, Harbeck N. Hurvitz SA, et al. Expert Opin Investig Drugs. 2014 Jul;23(7):1039-47. doi: 10.1517/13543784.2014.924505. Epub 2014 May 29. Expert Opin Investig Drugs. 2014. PMID: 24870559 Review. - Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials.
Li J, Fu F, Yu L, Huang M, Lin Y, Mei Q, Lv J, Wang C. Li J, et al. Breast Cancer Res Treat. 2020 Feb;180(1):21-32. doi: 10.1007/s10549-020-05528-2. Epub 2020 Jan 22. Breast Cancer Res Treat. 2020. PMID: 31970560 Review.
Cited by
- Engineered Age-Mimetic Breast Cancer Models Reveal Differential Drug Responses in Young and Aged Microenvironments.
Yang J, Hawthorne L, Stack S, Blagg B, Ali A, Zorlutuna P. Yang J, et al. bioRxiv [Preprint]. 2024 Oct 10:2024.10.06.616903. doi: 10.1101/2024.10.06.616903. bioRxiv. 2024. PMID: 39416111 Free PMC article. Preprint. - Edaravone: A Novel Possible Drug for Cancer Treatment?
Duranti E, Cordani N, Villa C. Duranti E, et al. Int J Mol Sci. 2024 Jan 29;25(3):1633. doi: 10.3390/ijms25031633. Int J Mol Sci. 2024. PMID: 38338912 Free PMC article. Review. - Recent Advances in Drug Discovery for Triple-Negative Breast Cancer Treatment.
Masci D, Naro C, Puxeddu M, Urbani A, Sette C, La Regina G, Silvestri R. Masci D, et al. Molecules. 2023 Nov 9;28(22):7513. doi: 10.3390/molecules28227513. Molecules. 2023. PMID: 38005235 Free PMC article. Review. - Molecular Biology Mechanisms and Emerging Therapeutics of Triple-Negative Breast Cancer.
Zhang Z, Zhang R, Li D. Zhang Z, et al. Biologics. 2023 Sep 21;17:113-128. doi: 10.2147/BTT.S426392. eCollection 2023. Biologics. 2023. PMID: 37767463 Free PMC article. Review. - Advancing Cancer Therapy Predictions with Patient-Derived Organoid Models of Metastatic Breast Cancer.
Önder CE, Ziegler TJ, Becker R, Brucker SY, Hartkopf AD, Engler T, Koch A. Önder CE, et al. Cancers (Basel). 2023 Jul 13;15(14):3602. doi: 10.3390/cancers15143602. Cancers (Basel). 2023. PMID: 37509265 Free PMC article.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. International Agency for Research on Cancer. http://globocan.iarc.fr. Accessed 4 July 2011
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. - DOI - PMC - PubMed
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. - DOI - PubMed
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. - DOI - PMC - PubMed
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous