A CXCL1 paracrine network links cancer chemoresistance and metastasis - PubMed (original) (raw)
. 2012 Jul 6;150(1):165-78.
doi: 10.1016/j.cell.2012.04.042.
Thordur Oskarsson, Sakari Vanharanta, Srinivas Malladi, Juliet Kim, Patrick G Morris, Katia Manova-Todorova, Margaret Leversha, Nancy Hogg, Venkatraman E Seshan, Larry Norton, Edi Brogi, Joan Massagué
Affiliations
- PMID: 22770218
- PMCID: PMC3528019
- DOI: 10.1016/j.cell.2012.04.042
A CXCL1 paracrine network links cancer chemoresistance and metastasis
Swarnali Acharyya et al. Cell. 2012.
Abstract
Metastasis and chemoresistance in cancer are linked phenomena, but the molecular basis for this link is unknown. We uncovered a network of paracrine signals between carcinoma, myeloid, and endothelial cells that drives both processes in breast cancer. Cancer cells that overexpress CXCL1 and 2 by transcriptional hyperactivation or 4q21 amplification are primed for survival in metastatic sites. CXCL1/2 attract CD11b(+)Gr1(+) myeloid cells into the tumor, which produce chemokines including S100A8/9 that enhance cancer cell survival. Although chemotherapeutic agents kill cancer cells, these treatments trigger a parallel stromal reaction leading to TNF-α production by endothelial and other stromal cells. TNF-α via NF-kB heightens the CXCL1/2 expression in cancer cells, thus amplifying the CXCL1/2-S100A8/9 loop and causing chemoresistance. CXCR2 blockers break this cycle, augmenting the efficacy of chemotherapy against breast tumors and particularly against metastasis. This network of endothelial-carcinoma-myeloid signaling interactions provides a mechanism linking chemoresistance and metastasis, with opportunities for intervention.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
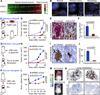
Figure 1. CXCL1/2 mediate mammary tumor growth and lung metastasis
(A) CXCL1 and CXCL2 expression in 615 primary breast cancers based on microarray gene expression datasets. Correlation between the two genes was determined by Pearson’s correlation coefficient (n=615, r=0.53, p<2.2e−16). P value was determined by Student’s t-test. (B) Breast cancer tissue microarray (TMA) samples composed of normal breast tissue, primary breast tumors and metastases (LN from lymph node and lung metastases) from patients analyzed by FISH. Signals in green correspond to 4q centromeric reference probe and signals in red correspond to CXCL1/2 probes. Scale bar, 2.5µm. (C) Schematic representation of breast cancer progression in orthotopic allograft model. PyMT mammary cancer cells were isolated from MMTV-PyMT mammary tumors, transduced with shRNA control or sh_Cxcl1/2_ and transplanted into syngeneic mice. (D) Growth curves of tumors from control and sh_Cxcl1/2_ PyMT-F cells. Data are averages ± SEM. n=6 mice per group. (E) Spontaneous lung metastasis determined by H&E staining of lung sections at week 9 after tumor inoculation at endpoint. Scale bars equal 10µm. (F) Quantitation of lung metastasis determined by automated counting of foci per field of view (FOV). Data are averages ± SEM. n=6 mice per group. P values were determined by Student’s t-test. (G) Schematic representation of breast cancer progression in orthotopic xenograft model. LM2 metastatic breast cancer cells were implanted into immunodeficient NOD-SCID mice. Mammary tumor growth and lung metastasis were determined. (H) Growth curves of tumors from LM2 cells transduced with control or CXCL1/2 shRNA. Data are averages ± SEM. Control n=13, sh_CXCL1/2_ n=7. (I) Representative histology images of spontaneous lung metastasis detected by vimentin immunostaining. Scale bars equal 60µm. (J) Quantitation of lung metastatic burden determined by automated counting of number of foci per FOV. Shown are averages ± SEM. n=5 mice per group. P values was determined by Student’s t-test. (K) Schematic representation of lung colonization assay in xenograft model. Luciferase labeled MDA231-LM2 cells transduced with control or sh_CXCL1/2_ were injected intravenously and monitored over time by non-invasive bioluminescence imaging (BLI). (L) BLI quantification of lung colonization ability of control or sh_CXCL1/2_ LM2 cells. Data are averages ± SEM. n=7 per group. P values were determined by Student’s t-test. (M) Representative BLI images of mice with lung metastasis (N) Cancer cells in the lungs stained for vimentin expression. See also Figure S1
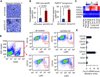
Figure 2. Carcinoma-derived CXCL1/2 supports cancer cell survival and recruit granulocytic myeloid cells to tumors
(A–B) Representative images and quantification of apoptosis in mammary tumors analyzed by Cleaved caspase-3 staining. Mouse mammary glands were injected with LM2 cells (A,B) or PyMT-F cells (B) expressing shRNA control or sh_CXCL1/2_ and analyzed at endpoint (LM2, 6 weeks; PyMT-F, 9 weeks after tumor implantation). Scale bar equals 30µm. Data are averages ± SEM. n=4 mice per group. P values were calculated by Student’s t-test. (C) Expression of the indicated genes from microarray gene expression analysis in non-tumor human mammary epithelial cell line MCF10A, parental MDA-MB-231 breast cancer cells and lung metastatic lines derived from MDA-MB-231 (Minn et al., 2005). (D) Flow cytometric analysis of recruited myeloid cells in tumors formed by LM2 cells transduced with either control shRNA or sh_CXCL1/2_ at 5 weeks after tumor inoculation. A representative gating is shown. Numbers indicate either CD11b+Ly6G+ or CD11b+Ly6C+ cells in the quadrant expressed as percentages of total CD45+ leukocytes from the same tumor. Results are representative of three independent experiments (n=3). (E) Expression of Cxcr2 receptor in sorted subpopulations of LM2 tumors determined by qRT-PCR. Error bars represent 95% confidence interval for qRT-PCR analysis. Data is representative of two independent experiments. See also Figure S2 and Tables S1 and S2
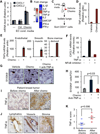
Figure 3. CXCL1/2 promote metastasis through myeloid cell-derived S100A8/9
(A–B) Gene ranking according to correlation with CXCL1 expression. Expression data from breast cancer primary and metastases microarray datasets. Genes were filtered based on extracellular localization to identify paracrine mediators. The list on the right shows genes that correlate highest with CXCL1. Complete list of CXCL1 correlating genes in Tables S3 and S4. (C) Expression of the top seven _CXCL1_-associated genes in (A) in the sorted CD11b+Gr1+ cells compared to unsorted tumor determined by qRT-PCR analysis from LM2 breast cancer model and MMTV-PyMT autochthonous mammary cancer model. Error bars represent 95% confidence interval. Data is representative of two independent experiments. (D) Expression of top seven _CXCL1_-associated genes in breast cancer cell lines based on microarray gene expression data. (E) LM2 tumor growth curves in mice transplanted with either S100a9+/+ or _S100a9_−/− bone marrow. Data points show averages ± SEM. n=19 tumors per group. P value was determined by Student’s t-test. (F–G) Representative images and quantitation of metastatic cells in lungs detected by vimentin immunohistochemistry at 60 days after inoculation of LM2 tumors, in mice that were transplanted with S100a9+/+ or S100a9_−/− bone marrow. Scale bar equals 60µm. Data points show averages ± SEM. n=4–6 mice per group. P value was determined by Student’s t-test. (H–I) Lung colonization by LM2 cells transduced with control shRNA or sh_CXCL1/2, with or without ectopic expression of S100a8/9. Lung colonization was assessed by non-invasive bioluminescence imaging (BLI) at 4 weeks after tail vein injection of the cells. (H) Normalized BLI quantification (I) images represented by photon flux of lung colonization ability. Data are averages ± SEM. n=4–6 per group. ns, not significant. P value was determined by two-tailed Wilcoxon Rank Sum test. (J) Representative TMA cores containing lung metastasis samples from breast cancer patients stained for total S100A8/9. (K) Kaplan-Meier overall survival analysis on breast cancer patients classified by total S100A8/9 expression in lung metastasis (see panel J) n=23 for S100A8/9 low group, n=17 for S100A8/9 high group. P-values were calculated by log-rank test. See also Figure S3 and Tables S3 and S4
Figure 4. S100A8/9 promotes breast cancer cell survival under chemotherapy
(A) Quantitation of apoptosis by cleaved caspase-3 staining in tumors and TUNEL assay in lungs in mice transplanted with S100a9+/+ or _S100a9_−/− bone marrow at 60 days after LM2 tumor inoculation into the mammary fat pad. Data are averages ± SEM. n=4–6 mice per group. P values were determined by Student’s t-test. (B–C) Representative images and quantification of apoptosis by cleaved caspase 3 staining in LM2 tumors from mice transplanted with either S100a9+/+ or _S100a9_−/− bone marrow and subsequently treated with a combination of doxorubicin and cyclophosphamide chemotherapy (AC regimen, chemo) once weekly for 3 weeks. Scale bar represents 32µm. Data are averages ± SEM. n=5–6 tumors per group. P values were determined by Student’s t-test. (D) TUNEL analysis detecting apoptotic cancer cells in co-culture assay. LM2 cancer cells were cultured alone or overnight in the presence of S100a9+/+ or _S100a9_−/− bone marrow cells and subsequently treated with chemotherapeutic drug (Chemo), doxorubicin (0.8µM). Data are average ± SEM of triplicates. P values determined by Student’s t-test. (E) Screening of signaling pathways activated by S100A8/9 in LM2 metastatic cancer cells by probing human phospho-kinase array with lysates from cells treated with either PBS or 10µg/ml recombinant S100A8/9. Shorter exposure of the same blot shown in S4. Proteins showing increased phosphorylation upon S100A8/9 treatment are highlighted. (F) TUNEL analysis detecting apoptotic cancer cells in co-culture assay upon pharmacological inhibition of p38, p70S6K and ERK pathways in the presence of recombinant S100A8/9. LM2 cancer cells were pretreated with 10µg/ml of S100A8/9 for 1 h and subsequently treated with doxorubicin (0.8µM, chemo) either alone or in the presence of 5µM of p38 inhibitor SB203580 or 10µM of p70S6K inhibitor PF4708671 or 10µM of ERK inhibitor FR180204 for 16h. LM2 cells treated with saline were used as controls. Quantification of apoptosis was done by calculating the percentage of TUNEL+/DAPI+ cells per FOV. Data are shown as average ± SEM from triplicates. *, **, ***, **** denote p=0.004, 0.02, 0.04, 0.01, respectively. P values were determined by Student’s t-test. See also Figure S4
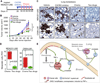
Figure 5. CXCL1/2 paracrine axis is hyperactivated upon chemotherapy treatment
(A) Tumor growth in mice treated with saline vehicle or a combination of doxorubicin and cyclophosphamide chemotherapy (AC chemo). The treatment was initiated once LM2 tumors reached 300 mm3 and was repeated once weekly. Data represent averages ± SEM. n=6–8 mice per group. P values were determined by Student’s t-test. (B) Apoptosis determined by TUNEL staining in tumors treated with vehicle or AC chemotherapy for 3 days (early) or 8 days (late) both using the same treatment regimen. Data represent averages ± SEM. n=3–5 mice per group. P values were calculated by Student’s t-test. *p=0.02, **p<0.0001. (C) CXCL1/2 expression in whole tumors harvested from mice treated with saline vehicle or AC chemotherapy for 8 days. Data represent averages±SEM. n=6–8 mice per group. P values were determined by Student’s t-test. (D) Representative CXCL1 expression in whole tumors analyzed by immunohistochemistry harvested from mice treated with saline vehicle or AC chemotherapy (prolonged treatment) from two independent cohorts of three mice each. Scale bar represents 70µm. (E) Quantitation of S100A9 positive cells in tumors from control and AC chemotherapy treated mice (prolonged treatment). Data presented are average numbers of S100A9 positive cells per FOV ± SEM. n=4–5 mice/group. Data representative of three independent experiments. (F) Expression of S100a8 and S100a9 in sorted subpopulations of LM2 tumors after chemotherapy treatment determined by qRT-PCR. Error bars represent 95% confidence interval. Data representative of two independent experiments. (G) Immunohistochemical analysis of S100A8/9 in tumors from breast cancer patients before and after AC chemotherapy treatment. Representative images of scored S100A8/9 sections are shown. Scale bar equals 60µm. (H) S100A8/9 expression score in paired patient tumor samples, before and after chemotherapy. Data represent expression score. n=40. P values determined by Wilcoxon’s paired test, comparing pre and post-treatment levels from each patient. See also Figure S5 and Table S5
Figure 6. TNF-α from chemotherapy-activated stroma boosts the CXCL1/2 survival axis
(A) CXCL1/2 expression in MDA231-LM2 cancer cells either alone (–) or in the presence of conditioned media from primary human umbilical vein endothelial cells (HUVEC) that were either untreated (control) or treated with 0.8µM doxorubicin (chemo), as determined by qRT-PCR. Data represent average expression ± SEM. (B) Heatmap representing expression changes in inflammatory cytokines in HUVEC treated with doxorubicin (0.8µM). Heatmap generated by conversion of qRT-PCR data that was normalized to β_2M_ as housekeeping gene control. (C) Schematic representation of experimental procedures. Lung endothelial cells were purified from LM2 tumor-bearing mice that were treated with chemotherapy. Mice showing established lung metastasis 7 weeks after tail-vein injection of LM2 cells were either treated with vehicle (saline) or AC chemotherapy. CD31+ endothelial cells were purified from dissociated lung tissue by flow cytometry. (D) TNF-α expression in isolated CD31+ lung endothelial cells from doxorubicin treated tumor-bearing mice. n=2–4 mice per group. Data represent averages ± SEM. (E) _TNF_-α expression in the indicated primary cells upon doxorubicin chemotherapy treatment for 16h as determined by qRT-PCR analysis. Error bars represent 95% confidence interval for qRT-PCR analysis. Data is representative of three independent experiments. (F) CXCL1 expression in LM2 cancer cells treated with vehicle or TNF-α for 2h in the presence of a 100µM NBD (NEMO-binding domain) inhibitory peptide of the NF-κB pathway. Data represent averages ± SEM. (G–H) Representative images of S100A9 expression by immunohistochemistry and quantitation of S100A9 positive cells in tumors from control or AC chemotherapy treated mice, with or without anti-TNF-α blocking antibody (infliximab). Mice bearing CN34LM1 tumors were treated once weekly for five weeks starting at 10 weeks after tumor inoculation, with PBS vehicle, AC chemotherapy (chemo), or chemotherapy plus anti-TNF-α antibody. Scale bar, 32µm. Data are averages ± SEM. n=3–5 mice/group. P values were calculated by Student’s t-test. (I) Immunohistochemical analysis of TNF-α expression in human primary breast tumor before and after chemotherapy treatment. Scale bar equals 120µm. (J) Stromal rich areas containing lymphatic vessels, blood vessels and fibroblasts with high TNF-α staining from primary breast tumors after AC chemotherapy treatment. Magnified fields from images taken at 40× magnification. (K) Comparison of stromal TNF-α expression score in paired breast tumors before and after chemotherapy. n=8 patients. P value was determined by Wilcoxon’s paired test, comparing pre and post-treatment levels from each patient. See also Figure S6
Figure 7. Pharmacological inhibition of CXCL1 signaling sensitizes cancer cells to chemotherapy in metastatic breast cancer
(A–B) Schematic treatment flow (A), and tumor growth (B) of LM2 tumors in mice treated with PEG vehicle or CXCR2 inhibitor for the indicated periods (blue boxes) and treatment with saline vehicle or AC chemotherapy at the indicated days (red arrows). Data represent average expression ± SEM. n=10–13 mice per group. P values were determined by Student’s t-test. *p=0.02, **p=0.007. (C–D) Lung metastasis in MDA231-LM2 and CN34-LM1 orthotopic xenograft models undergoing treatment (C) Representative images of lung sections stained for vimentin expression marking metastatic cancer cells. Scale bars equal 100µm. (D) Quantitation of metastasis based on number of cancer cells in lung sections. Data are average foci per FOV ± SEM. n=5–10 mice per group. Whiskers represent minimum and maximum values. P values were determined by two-tailed Wilcoxon rank-sum test. (E) Model showing how CXCL1 paracrine interactions promote resistance to chemotherapy and metastasis in breast tumors and lung microenvironment. Genotoxic agents such as doxorubicin, cyclophosphamide and paclitaxel limit the survival of cancer cells but also increase TNF-α production from endothelial cells. TNF-α enhances CXCL1/2 expression in cancer cells. Other modes of CXCL1/2 upregulation in cancer cells include 4q21 amplification and overexpression. CXCL1/2 from cancer cells recruit CD11b+Gr1+ myeloid cells that express CXCR2 (receptors for CXCL1/2). Myeloid cells recruited by CXCL1/2 thereby enhance viability of cancer cells through S100A8/9 factors. See also Figure S7
Comment in
- Domino effect.
Seton-Rogers S. Seton-Rogers S. Nat Rev Cancer. 2012 Jul 24;12(8):506. doi: 10.1038/nrc3332. Nat Rev Cancer. 2012. PMID: 22825210 No abstract available. - Cell signalling: Stuck in the middle of chemoresistance and metastasis.
Villanueva MT. Villanueva MT. Nat Rev Clin Oncol. 2012 Sep;9(9):490. doi: 10.1038/nrclinonc.2012.129. Epub 2012 Jul 31. Nat Rev Clin Oncol. 2012. PMID: 22850753 No abstract available.
Similar articles
- Tumor-associated macrophages/C-X-C motif chemokine ligand 1 promotes breast cancer autophagy-mediated chemoresistance via IGF1R/STAT3/HMGB1 signaling.
Yang B, Li G, Wang S, Zheng Y, Zhang J, Pan B, Wang N, Wang Z. Yang B, et al. Cell Death Dis. 2024 Oct 11;15(10):743. doi: 10.1038/s41419-024-07123-5. Cell Death Dis. 2024. PMID: 39394189 Free PMC article. - CXCL1 from tumor-associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin β1/FAK/AKT signaling.
Wang Z, Wang Z, Li G, Wu H, Sun K, Chen J, Feng Y, Chen C, Cai S, Xu J, He Y. Wang Z, et al. Cancer Lett. 2017 Jan 28;385:28-38. doi: 10.1016/j.canlet.2016.10.043. Epub 2016 Nov 8. Cancer Lett. 2017. PMID: 27832972 - Benzyl butyl phthalate increases the chemoresistance to doxorubicin/cyclophosphamide by increasing breast cancer-associated dendritic cell-derived CXCL1/GROα and S100A8/A9.
Hsu YL, Hung JY, Tsai EM, Wu CY, Ho YW, Jian SF, Yen MC, Chang WA, Hou MF, Kuo PL. Hsu YL, et al. Oncol Rep. 2015 Dec;34(6):2889-900. doi: 10.3892/or.2015.4307. Epub 2015 Sep 23. Oncol Rep. 2015. PMID: 26397389 - S100A8/A9 activate key genes and pathways in colon tumor progression.
Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. Ichikawa M, et al. Mol Cancer Res. 2011 Feb;9(2):133-48. doi: 10.1158/1541-7786.MCR-10-0394. Epub 2011 Jan 12. Mol Cancer Res. 2011. PMID: 21228116 Free PMC article. - The Clinical Significance and Involvement in Molecular Cancer Processes of Chemokine CXCL1 in Selected Tumors.
Korbecki J, Bosiacki M, Szatkowska I, Kupnicka P, Chlubek D, Baranowska-Bosiacka I. Korbecki J, et al. Int J Mol Sci. 2024 Apr 15;25(8):4365. doi: 10.3390/ijms25084365. Int J Mol Sci. 2024. PMID: 38673949 Free PMC article. Review.
Cited by
- Non-homogenous intratumor ionizing radiation doses synergize with PD1 and CXCR2 blockade.
Bergeron P, Dos Santos M, Sitterle L, Tarlet G, Lavigne J, Liu W, Gerbé de Thoré M, Clémenson C, Meziani L, Schott C, Mazzaschi G, Berthelot K, Benadjaoud MA, Milliat F, Deutsch E, Mondini M. Bergeron P, et al. Nat Commun. 2024 Oct 14;15(1):8845. doi: 10.1038/s41467-024-53015-9. Nat Commun. 2024. PMID: 39397001 Free PMC article. - Tumor-associated macrophages/C-X-C motif chemokine ligand 1 promotes breast cancer autophagy-mediated chemoresistance via IGF1R/STAT3/HMGB1 signaling.
Yang B, Li G, Wang S, Zheng Y, Zhang J, Pan B, Wang N, Wang Z. Yang B, et al. Cell Death Dis. 2024 Oct 11;15(10):743. doi: 10.1038/s41419-024-07123-5. Cell Death Dis. 2024. PMID: 39394189 Free PMC article. - Senescent cells promote breast cancer cells motility by secreting GM-CSF and bFGF that activate the JNK signaling pathway.
Wang N, Fang Y, Hou Y, Cheng D, Dressler EV, Wang H, Wang J, Wang G, Li Y, Liu H, Xiang R, Yang S, Sun P. Wang N, et al. Cell Commun Signal. 2024 Oct 7;22(1):478. doi: 10.1186/s12964-024-01861-x. Cell Commun Signal. 2024. PMID: 39375718 Free PMC article. - The expression patterns of different cell types and their interactions in the tumor microenvironment are predictive of breast cancer patient response to neoadjuvant chemotherapy.
Dhruba SR, Sahni S, Wang B, Wu D, Rajagopal PS, Schmidt Y, Shulman ED, Sinha S, Sammut SJ, Caldas C, Wang K, Ruppin E. Dhruba SR, et al. bioRxiv [Preprint]. 2024 Jun 14:2024.06.14.598770. doi: 10.1101/2024.06.14.598770. bioRxiv. 2024. PMID: 39372749 Free PMC article. Preprint. - ACSL6-activated IL-18R1-NF-κB promotes IL-18-mediated tumor immune evasion and tumor progression.
Di Y, Wang Z, Xiao J, Zhang X, Ye L, Wen X, Qin J, Lu L, Wang X, He W. Di Y, et al. Sci Adv. 2024 Sep 20;10(38):eadp0719. doi: 10.1126/sciadv.adp0719. Epub 2024 Sep 18. Sci Adv. 2024. PMID: 39292786 Free PMC article.
References
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. - PubMed
- Bieche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L, Guinebretiere JM, Burlinchon S, Lidereau R, Lazennec G. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer. 2007;14:1039–1052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- U54 CA163167/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- CA94060/CA/NCI NIH HHS/United States
- P01 CA094060/CA/NCI NIH HHS/United States
- R37 CA034610/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials