Phage morphology recapitulates phylogeny: the comparative genomics of a new group of myoviruses - PubMed (original) (raw)
Phage morphology recapitulates phylogeny: the comparative genomics of a new group of myoviruses
André M Comeau et al. PLoS One. 2012.
Abstract
Among dsDNA tailed bacteriophages (Caudovirales), members of the Myoviridae family have the most sophisticated virion design that includes a complex contractile tail structure. The Myoviridae generally have larger genomes than the other phage families. Relatively few "dwarf" myoviruses, those with a genome size of less than 50 kb such as those of the Mu group, have been analyzed in extenso. Here we report on the genome sequencing and morphological characterization of a new group of such phages that infect a diverse range of Proteobacteria, namely Aeromonas salmonicida phage 56, Vibrio cholerae phages 138 and CP-T1, Bdellovibrio phage φ1422, and Pectobacterium carotovorum phage ZF40. This group of dwarf myoviruses shares an identical virion morphology, characterized by usually short contractile tails, and have genome sizes of approximately 45 kb. Although their genome sequences are variable in their lysogeny, replication, and host adaption modules, presumably reflecting differing lifestyles and hosts, their structural and morphogenesis modules have been evolutionarily constrained by their virion morphology. Comparative genomic analysis reveals that these phages, along with related prophage genomes, form a new coherent group within the Myoviridae. The results presented in this communication support the hypothesis that the diversity of phages may be more structured than generally believed and that the innumerable phages in the biosphere all belong to discrete lineages or families.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
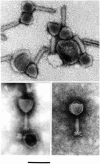
Figure 1. EM micrographs showing the morphology typical of the φPLPE group phages.
Presented are Aeromonas phage 56 (top, uranyl acetate), Bdellovibrio phage φ1422 (bottom left, phosphotungstate) and Vibrio phage 138 (bottom right, phosphotungstate). The scale bar is 100 nm and applies to all micrographs.
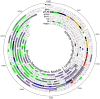
Figure 2. Comparative genomics of the eight φPLPE group phages.
Note that the previously published sequences of φPLPE and PY100 have been re-cut to a similar organization as Aaφ23. Gene abbreviations/functions are as follows: acylase, homoserine lactone acylase; ant, anti-repressor; atQ, anti-termination; bet, lambda recombination; BP, baseplate; cI/II, repressor; cro, anti-repressor; dnaC, replication; exo, exonuclease; H, head; hel, helicase; HNH, HNH (homing) endonuclease; hol, holin; int, integrase; lys, lysis; lyz, lysozyme; MCP, major capsid protein; meth, methylase; ninB/C/G, lambda recombination; nuc, nuclease; P, portal; pnk, polynucleotide kinase; pol, DNA polymerase; prim, primase; rec(T), recombination; recE, exonuclease VIII; rep(O/P), (λ) replication; Rz/Rz1, lysis; ssb, single-stranded binding; σ54, bacterial transcriptional regulator; T, tail; terS/L, terminase; Tu, elongation factor; tetR, bacterial transcriptional regulator; trans, transposase; V, virion; xis, excisionase.
Figure 3. Bipartite nature of the φPLPE group phage genomes, with variable lysogeny/replication modules and conserved structure/morphogenesis modules.
With the exception of φPLPE itself (all ORFs colored), only those ORFs shared with φPLPE in the other phages are color-coded as in Fig. 2. Shared ORFs were defined as protein matches in each phage against a φPLPE-restricted BLASTp with an _E_-value <10−4.
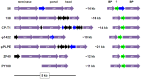
Figure 4. The two conserved structural mini-modules in the φPLPE group phages, excluding Aaφ23.
Color-coding is as in Fig. 2 and gene numbers refer to the φPLPE genome. ORF60 (“60″) is of unknown function, but could be implicated in the baseplate (BP). The two small ORFs upstream of the terL genes in φ1422 and ZF40 could be the terS genes. The terS/L genes in PY100 are not arranged as in the other phages and are far upstream and not side-by-side. All of the cellular hits shown are (conserved) hypothetical bacterial proteins, except for φ1422 which has a σ54 transcription regulator (“σ”).
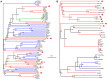
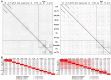
Figure 5. Neighbor-joining trees of TerL (A) and portal proteins (B; φPLPE gp19 homologs).
The eight dwarf φPLPE-like myoviruses are highlighted with red arrows. Branches are colored according to phage family type: red for Myoviruses, blue for Siphoviruses, green for Podoviruses and black for unknown morphology. Values at the nodes are the results of 100 bootstrap replicates. The scale bar indicates 0.1 substitutions per site.
Figure 6. Whole genome similarities among φPLPE group phages.
(A) Reciprocal dot-plots of the φPLPE-like phages based on whole genome nucleotide sequences (left) or concatenations of all proteins (right). Two Mu-like and two P2-like phages have been included for comparison. (B) Similarity matrices of the DNA sequences (left) and concatenated polyproteins (right) of the phages in (A). The non-φPLPE-like phages and a randomized sequence of φPLPE serve as controls. Also included are the results of the statistical tests comparing the similarity values of the φPLPE-like phages to the controls. Similarity values are highlighted with increasingly darker shades of red.
Similar articles
- Identification of the major proteins of the virions of bacteriophage ZF40 Pectobacterium carotovorum.
Korol NA, Tovkach FI. Korol NA, et al. Mikrobiol Z. 2012 Jul-Aug;74(4):64-70. Mikrobiol Z. 2012. PMID: 23088102 - Molecular Analysis of Arthrobacter Myovirus vB_ArtM-ArV1: We Blame It on the Tail.
Kaliniene L, Šimoliūnas E, Truncaitė L, Zajančkauskaitė A, Nainys J, Kaupinis A, Valius M, Meškys R. Kaliniene L, et al. J Virol. 2017 Mar 29;91(8):e00023-17. doi: 10.1128/JVI.00023-17. Print 2017 Apr 15. J Virol. 2017. PMID: 28122988 Free PMC article. - Comparative genomic analysis of dwarf Vibrio myoviruses defines a conserved gene cluster for successful phage infection.
Skliros D, Karpouzis E, Kalloniati C, Katharios P, Flemetakis E. Skliros D, et al. Arch Virol. 2022 Feb;167(2):501-516. doi: 10.1007/s00705-021-05340-3. Epub 2022 Jan 8. Arch Virol. 2022. PMID: 35000006 - The SPO1-related bacteriophages.
Klumpp J, Lavigne R, Loessner MJ, Ackermann HW. Klumpp J, et al. Arch Virol. 2010 Oct;155(10):1547-61. doi: 10.1007/s00705-010-0783-0. Epub 2010 Aug 17. Arch Virol. 2010. PMID: 20714761 Review. - Enterococcal Bacteriophages and Genome Defense.
Duerkop BA, Palmer KL, Horsburgh MJ. Duerkop BA, et al. 2014 Feb 11. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet]. Boston: Massachusetts Eye and Ear Infirmary; 2014–. 2014 Feb 11. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet]. Boston: Massachusetts Eye and Ear Infirmary; 2014–. PMID: 24649501 Free Books & Documents. Review.
Cited by
- Interference-driven spacer acquisition is dominant over naive and primed adaptation in a native CRISPR-Cas system.
Staals RH, Jackson SA, Biswas A, Brouns SJ, Brown CM, Fineran PC. Staals RH, et al. Nat Commun. 2016 Oct 3;7:12853. doi: 10.1038/ncomms12853. Nat Commun. 2016. PMID: 27694798 Free PMC article. - CRISPRTarget: bioinformatic prediction and analysis of crRNA targets.
Biswas A, Gagnon JN, Brouns SJ, Fineran PC, Brown CM. Biswas A, et al. RNA Biol. 2013 May;10(5):817-27. doi: 10.4161/rna.24046. Epub 2013 Mar 14. RNA Biol. 2013. PMID: 23492433 Free PMC article. - Four Escherichia coli O157:H7 phages: a new bacteriophage genus and taxonomic classification of T1-like phages.
Niu YD, McAllister TA, Nash JH, Kropinski AM, Stanford K. Niu YD, et al. PLoS One. 2014 Jun 25;9(6):e100426. doi: 10.1371/journal.pone.0100426. eCollection 2014. PLoS One. 2014. PMID: 24963920 Free PMC article. - Vibriophages and their interactions with the fish pathogen Vibrio anguillarum.
Tan D, Gram L, Middelboe M. Tan D, et al. Appl Environ Microbiol. 2014 May;80(10):3128-40. doi: 10.1128/AEM.03544-13. Epub 2014 Mar 7. Appl Environ Microbiol. 2014. PMID: 24610858 Free PMC article. - Novel Moraxella catarrhalis prophages display hyperconserved non-structural genes despite their genomic diversity.
Ariff A, Wise MJ, Kahler CM, Tay CY, Peters F, Perkins TT, Chang BJ. Ariff A, et al. BMC Genomics. 2015 Oct 24;16:860. doi: 10.1186/s12864-015-2104-1. BMC Genomics. 2015. PMID: 26497500 Free PMC article.
References
- Ackermann H-W. 5500 Phages examined in the electron microscope. Arch Virol. 2006;152:227–243. - PubMed
- Leblanc C, Caumont-Sarcos A, Comeau AM, Krisch HM. Isolation and genomic characterization of the first phage infecting Iodobacteria: φPLPE, a myovirus having a novel set of features. Environ Microbiol Rep. 2009;1:499–509. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous